Question
Question: Esters are functional isomers of: (A) Hydroxyaldehyde (B) Ketone (C) Diketone (D) Diol...
Esters are functional isomers of:
(A) Hydroxyaldehyde
(B) Ketone
(C) Diketone
(D) Diol
Solution
Isomerism is a phenomenon of organic chemistry in which more than one species has the same chemical formula but different structures. Those compounds who have same chemical formula but different properties and the arrangement of atoms in their molecule are called isomers
Complete answer:
Isomerism is of two types (a) Structural and (b) Stereo. Functional isomerism is a type of structural isomerism. Those molecules that have the same molecular formula but have different functional groups in their compounds are called functional isomers and this phenomenon is called functional isomerism.
Esters are produced when the carboxylic acid is reacted with alcohol in the presence of a catalyst. Concentrated sulphuric acid is used as a in this reaction. This reaction is called the esterification reaction. The general formula of esters is R−CO−OR′.
Hydroxycarbonyl compounds are the compounds that have a hydroxyl (−OH) group on the carbonyl carbon of aldehyde or ketone. Their general formula is
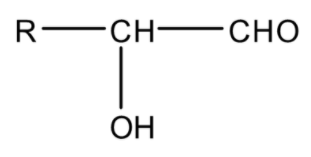 OR
OR 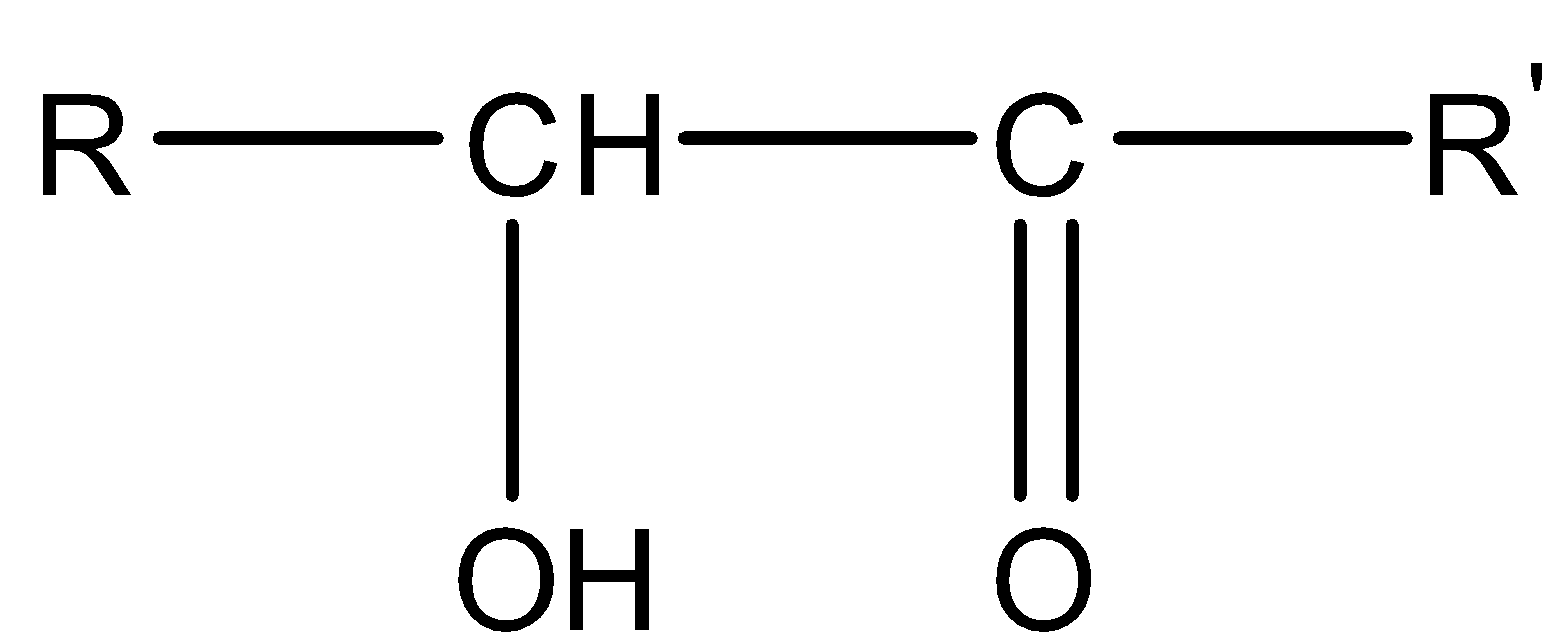
Take the example of Methyl ethanoate and 2− hydroxy propanal. Both have molecular formula C3H6O2 and their structure will be:
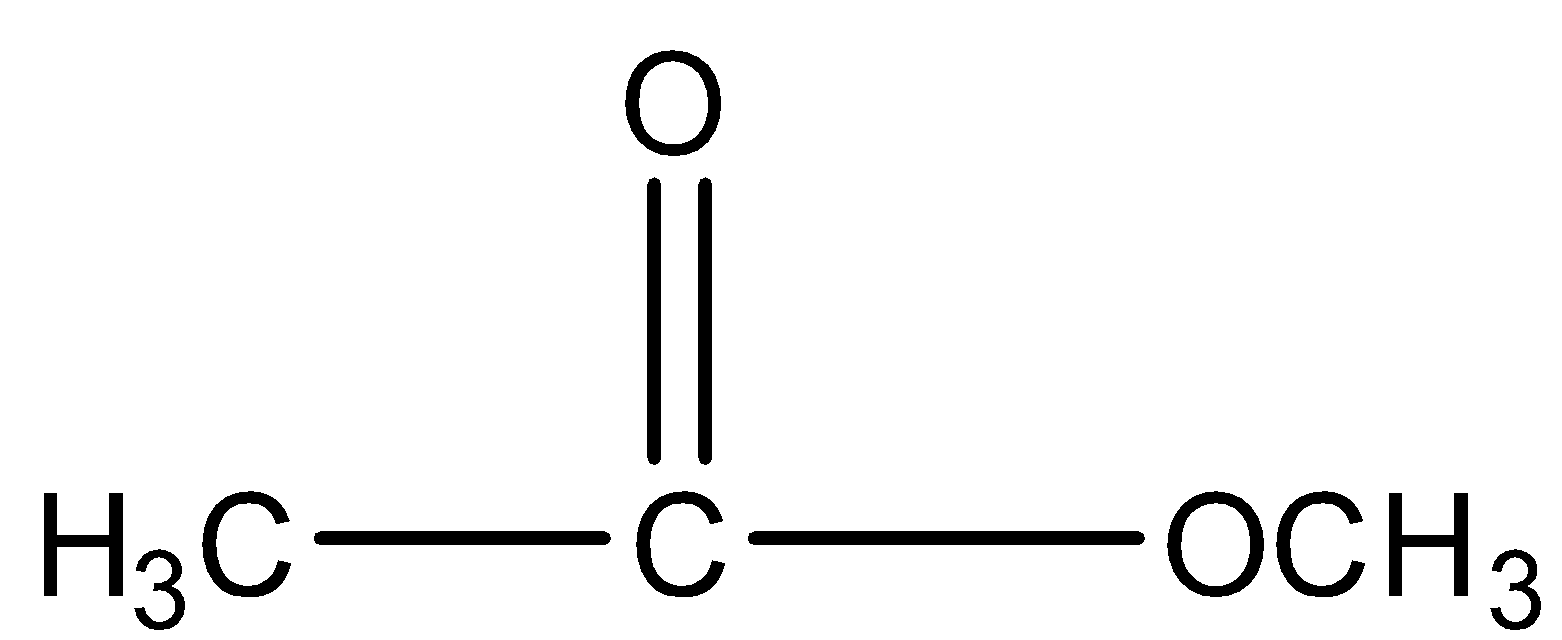 and
and 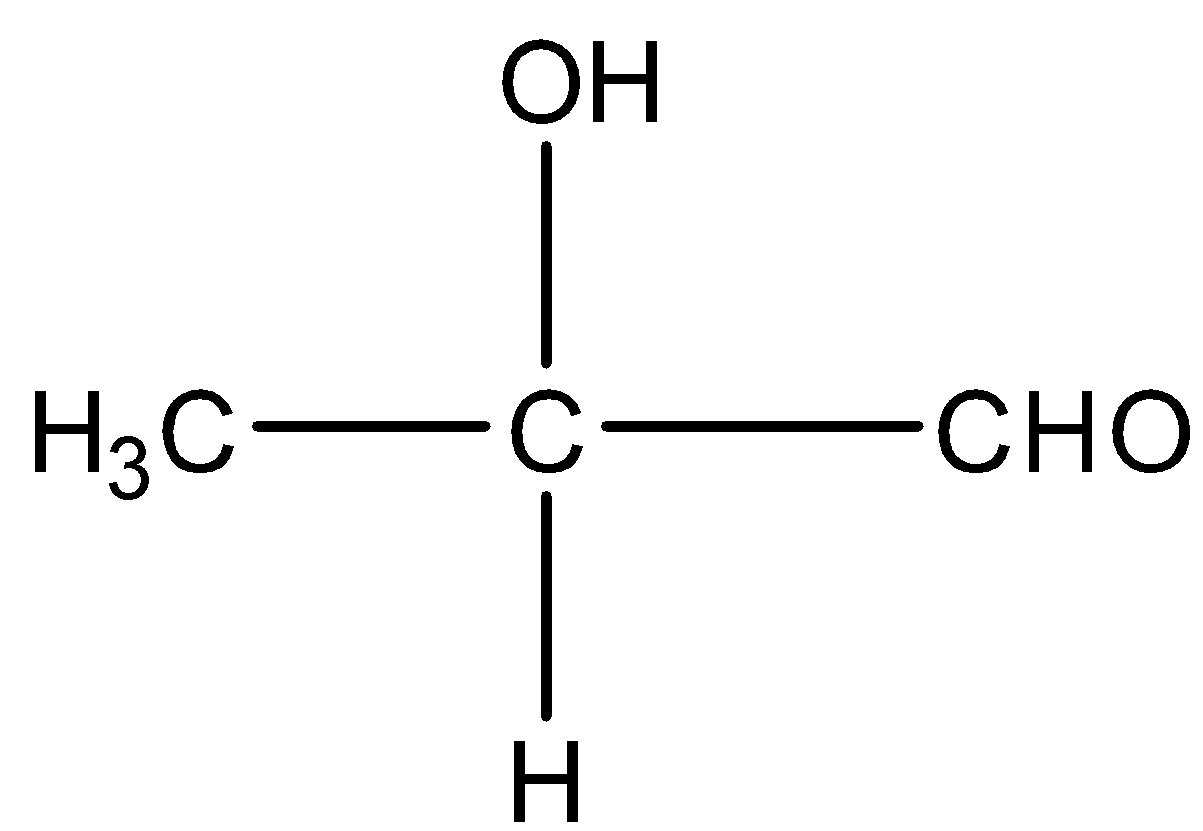
From these two compounds, we can deduce that the molecular formula is the same but the functional group is different. Hence, these are functional isomers of each other.
Monocarboxylic acids are also functional isomers of esters because both have the same molecular formula but different functional groups. For example; methyl ethanoate and propanoic acid both have chemical formula C3H6O2 but their chemical structure is different.
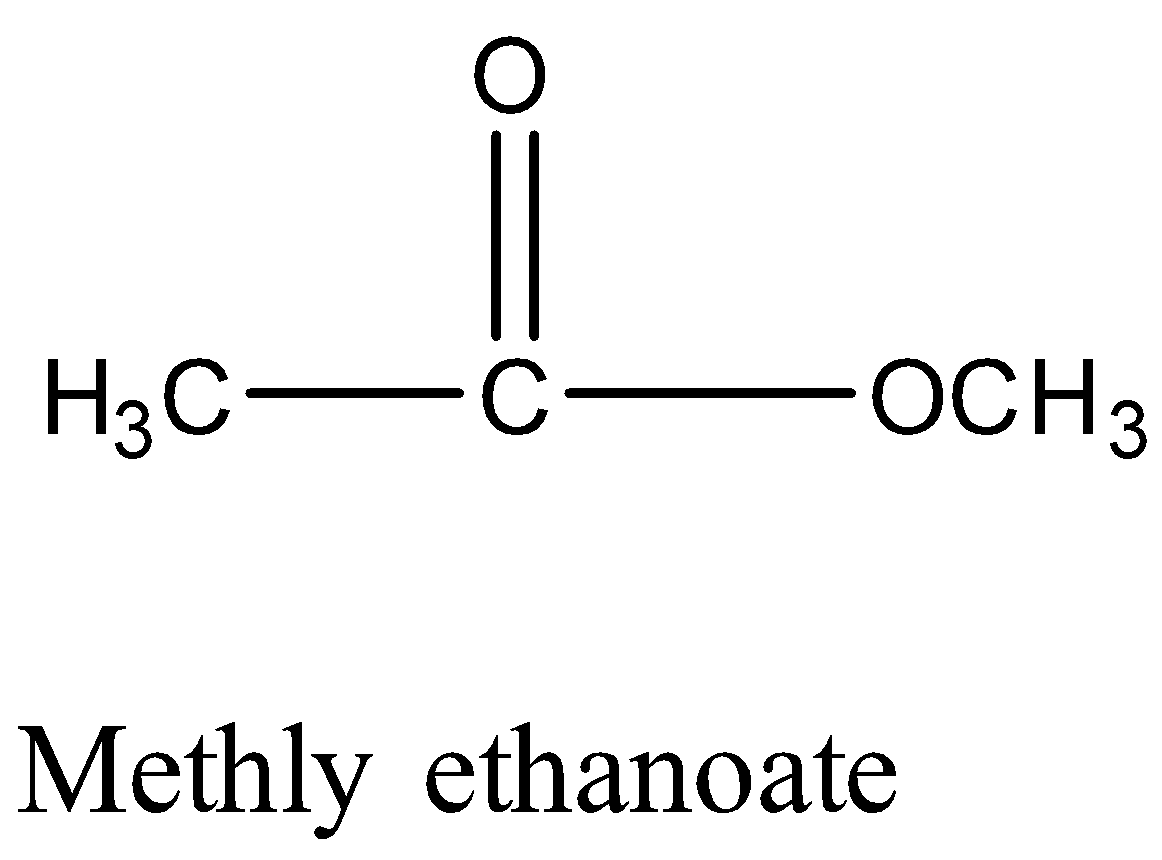
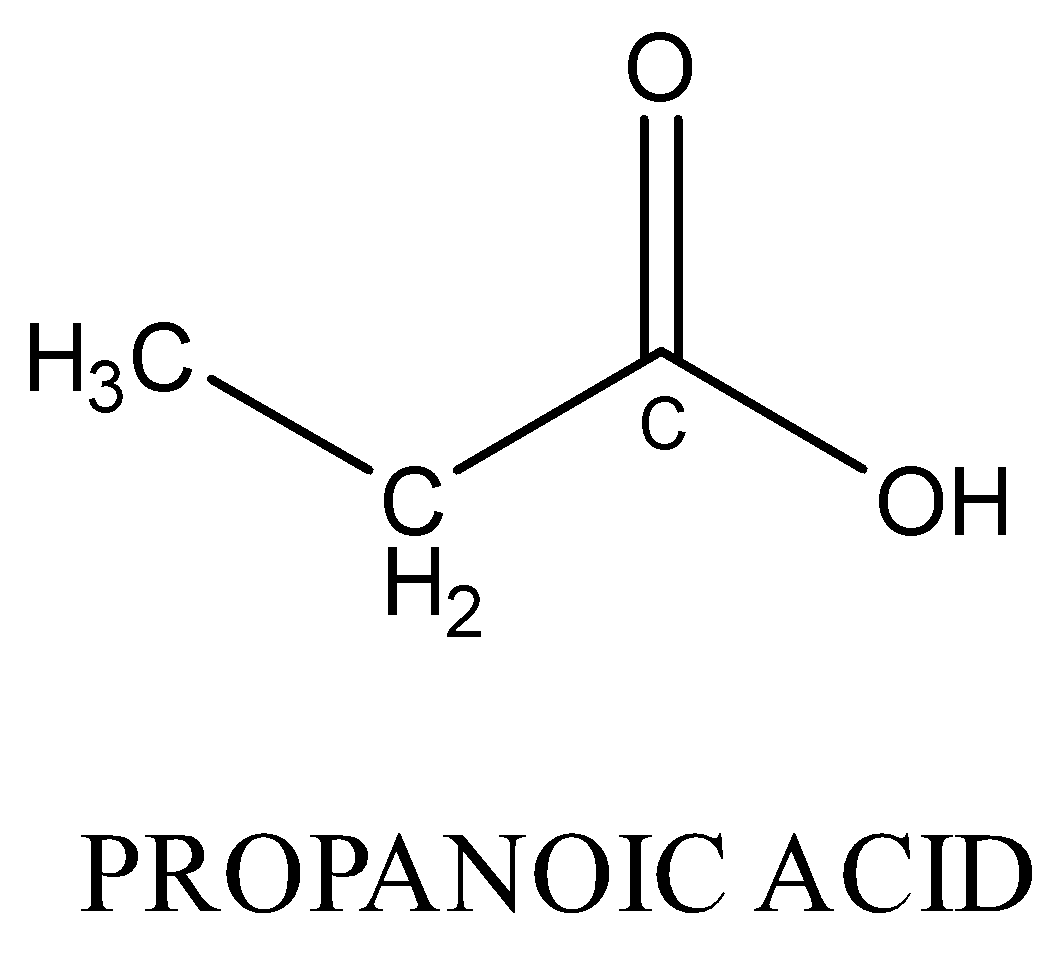
Therefore, Option (A) is correct.
Note:
So, The Functional isomers of esters are Hydroxycarbonyl compounds and carboxylic acids The functional group in carboxylic acid is −COOH, in esters it is R−CO−OR′. Both have the same molecular formula but different functional groups.
