Question
Question: Equivalent weight of \( M{n^{3 + }} \) in the following reaction is ( \( Mn = 55 \) ): \( M{n^{3 ...
Equivalent weight of Mn3+ in the following reaction is ( Mn=55 ):
Mn3+Mn2++MnO2
(A) 27.5
(B) 55
(C) 110
(D) 165
Solution
Here, a redox reaction is given and we have to calculate the equivalent weight of Mn3+ in the given reaction. So, first of all we have to understand the equivalent weight as it is the molar mass of a substance divided by the number of equivalents in the substance. Here, molar mass of the element Mn=55 is given. Using this we can calculate the equivalent weight.
Complete answer:
Here, the disproportionate reaction is given in the question so we have to balance this reaction as below:
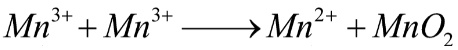
The half oxidation and reduction reactions are given below as:
oxidation reaction: Mn3++2H2O→MnO2+4H++1e
reduction reaction: Mn3++1e→Mn2+
Now the molar mass of Mn is 55g(mole)−1
Let us use the formula for equivalent weight for half oxidation reaction as:
Equivalent weight of oxidising agent = number of electron gained by one moleculemolar mass of Mn
Therefore,
Equivalent weight of oxidising agent = 155=55g.eq
Now for other half reduction reaction the formula for equivalent weight is:
Equivalent weight of reducing agent = number of electrons lost by one moleculemolar mass of Mn
Therefore,
Equivalent weight of reducing agent = 155 =55g.eq
So, the equivalent weight of Mn3+ is calculated as:
Equivalent weight of Mn3+=Equivalent weight of oxidising agent+Equivalent weight of reducing agent
∴Equivalent weight of Mn3+=(55+55)g.eq
∴Equivalent weight of Mn3+=110g.eq
Thus, we have calculated the equivalent weight of the Mn3+ as 110g.eq
Hence, the correct answer is option C.
Note:
Here, we have to understand the meaning of the equivalent weight as it is the molar mass of a substance divided by the number of equivalents in the substance and also that the reaction is redox as well as unbalanced. So we have to balance and also understand that we can be able to write it in half oxidation and half reduction reaction. We know that the gain of electrons represents oxidation and loss of electrons represents reduction reaction.
