Question
Question: Equivalent weight of hydrated oxalic acid is: A.\(44\) B.\(45\) C.\(63\) D.\(126\)...
Equivalent weight of hydrated oxalic acid is:
A.44
B.45
C.63
D.126
Solution
We have to remember that the oxalic acid is an organic compound which generally exists as a white colored solid crystalline form. And it forms a colorless solution in water. Oxalic acid occurs as a dihydrate containing two water molecules. We have to know that the oxalic acid is represented by the formula - C2H2O4. We have to know that the oxalic acids have acid strength greater than acetic acid.
Complete step by step answer:
We can draw the chemical structure of oxalic acid as,
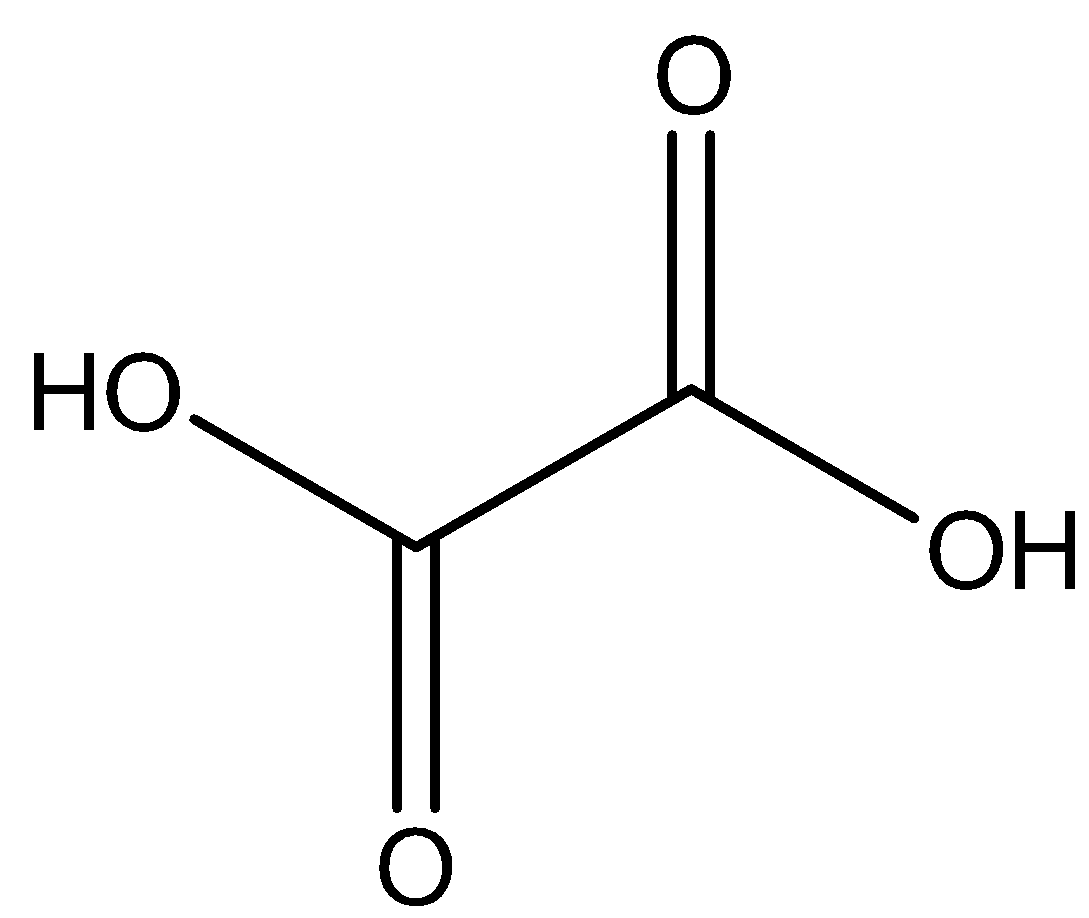
We know that is generally as dihydrate, its chemical formula can be represented as C2H2O4.2H2O.
To determine the equivalent weight, we will first divide the molecular weight with the n-factor of the given molecule. The n-factor is defined as the number of H+ ions replaced by 1mol of acid in a reaction and the number of OH− replaced by 1mol of base in a reaction.
Oxalic acid is acidic in nature.
Also, Molecular mass of hydrate oxalic acid is calculated as,
2C+6H+5O
Molecular mass =(2×12)+(6×1)+(6×16)=126
Since, the oxalic acid has two moles of acidic H+ , that means one mole of oxalic acid can neutralize two moles of OH− (base).
Hence, the number of equivalent moles is 2.
The formula of equivalent weight is given by,
Number of equivalent molesMolecular mass
Now we can substitute the known values we get,
=2126
On simplification we get,
Equivalent weight=63g/eq mol
Hence, the correct answer is option C.
Note:
We need to know that the oxalic acid is a strong reducing agent and its conjugate base is known as oxalate (when we remove the hydrogen ions from the formula).The concept of equivalent weight has dimensions and units of mass. It is generally defined as the mass of one equivalent of a given substance which will combine with or displace a fixed quantity of another substance. For acid-base reactions, the substituting element is the acidic and the basic ions which are involved in the reaction.
