Question
Question: Equivalent mass of sugar is: (A) \(\dfrac{M}{{11}}\) (B) \(\dfrac{M}{{12}}\) (C) \(\dfrac{M}{{...
Equivalent mass of sugar is:
(A) 11M
(B) 12M
(C) 22M
(D) None of these
Solution
Equivalent mass or gram equivalent is mass if one equivalent which will combine with another substance in a chemical reaction the equivalent mass of a compound can be calculated by cajoling the molecular mass of compound with number of electrons gained or lost.
Complete step by step answer:
Sugar is a carbohydrate with a churl call formula C12H22O11 It is also known as serosa cane or beet root.
Equivalent mass of sugar can be calculated by using the formula:-
Equivalent mass =numberofelectronMolecularMass gained or lost.
Sucrose is a disaccharide of glucose and fructose its structure is
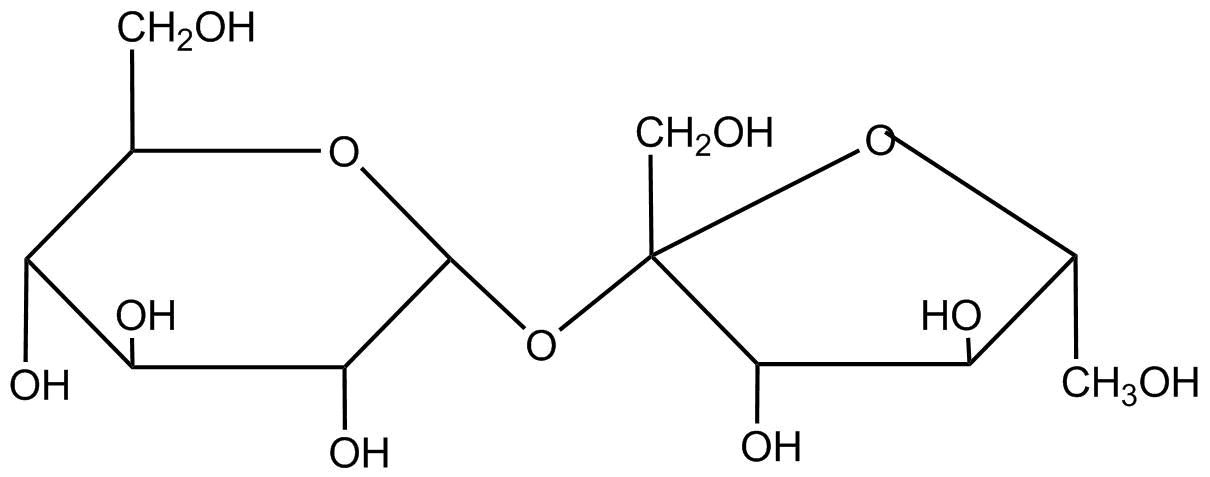
When sucrose undergoes a carnival reaction it gets reduced in it as loss of hydrogen’s present in it. As according to its formulaC12H22O11, sucrose has 22 hydrogen atoms. Therefore, it loses 22 hydro-gent atoms.
Therefore equivalent mass of sucrose is:-
Equivalent mass =22MolecularMass=22M
Note:- the actual equivalent mass of sugar is equivalent mass =numberofelectronsmolecularmassgained or lost.
Molecular mass of C12H22O11=12(12)+22(1)+11(16)=342G
Equivalent mass 22342=15.54g
Hence the correct option is C.
Note:
There are various types of Sugar
Fructose: found in fruits and honey.
Galactose: found in milk and dairy products.
Glucose: found in honey, fruits and vegetables.
Lactose: found in milk, made from glucose and galactose.
Maltose: found in barley.
Sucrose: made up of glucose and fructose and found in plants.
Xylose: found in wood or straw.
