Question
Question: Energy required to dissociate 16 g $O_{2(g)}$ into free atoms is $x$ kJ. The value of bond enthalpy ...
Energy required to dissociate 16 g O2(g) into free atoms is x kJ. The value of bond enthalpy of O=O bond is _______.
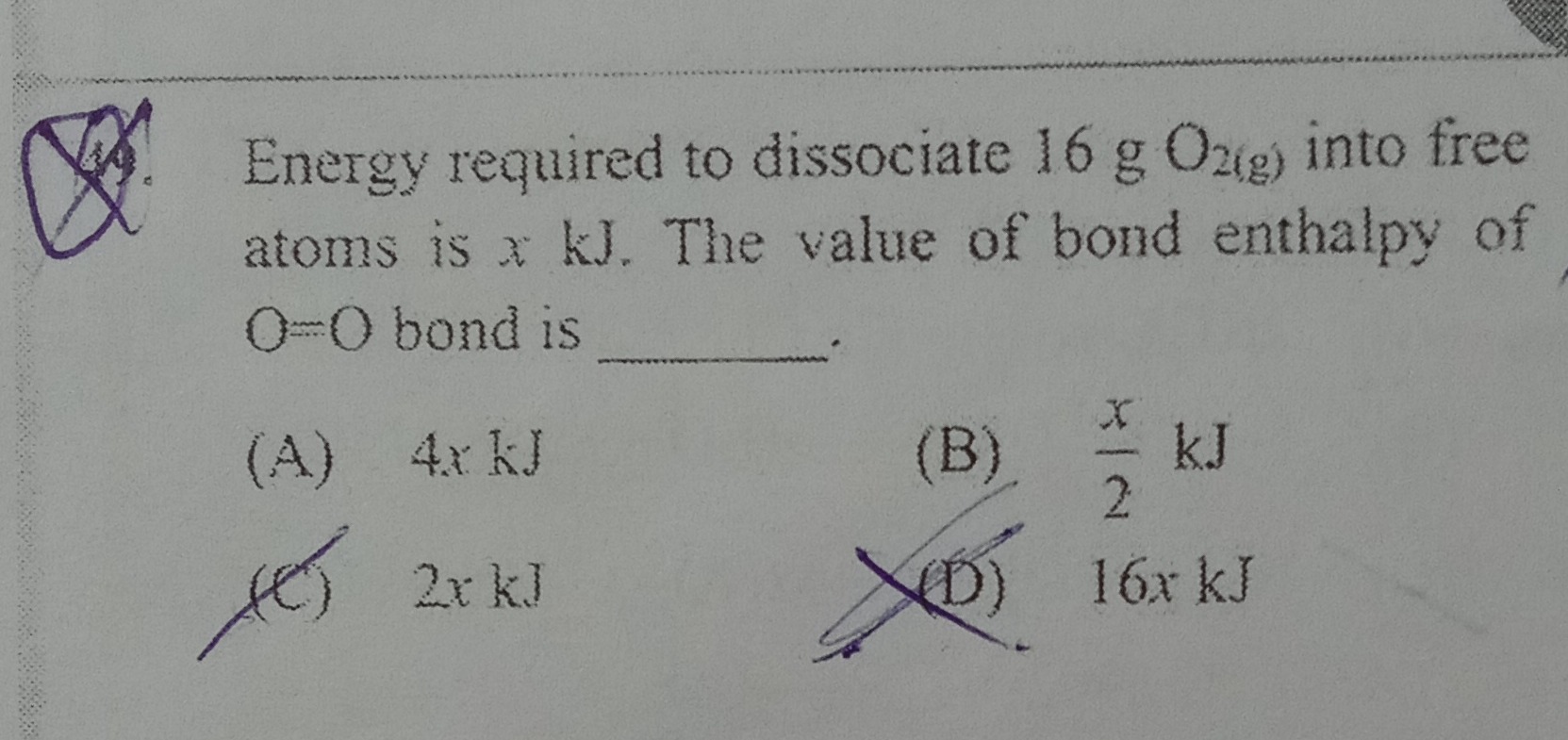
A
4x kJ
B
2x kJ
C
2x kJ
D
16x kJ
Answer
2x kJ
Explanation
Solution
Molecular mass of O2 = 32 g/mol, so 16 g of O2 = 0.5 mole. If x kJ dissociates 0.5 mole of O2 then energy required per mole (bond enthalpy) is
Bond enthalpy=0.5x=2x kJ/mol.
