Question
Question: Endosmosis takes place when a plant cell is immersed in a. Isotonic solution b. Hypotonic soluti...
Endosmosis takes place when a plant cell is immersed in
a. Isotonic solution
b. Hypotonic solution
c. Hypertonic solution
d. HCl solution
Solution
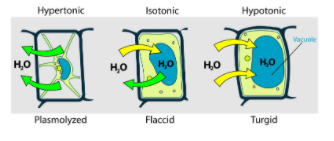
Endosmosis is a type of passive transport where the water moves from outside medium to inside of the cell. In a hypotonic solution, the concentration of the solvent (water) is high in the outside medium and solute concentration is high inside the cell due to this, solvent (water) enters the cell and increases turgidity.
Complete answer :
Endosmosis is defined as the passage of water from the region of high concentration to the region of low concentration via a semipermeable membrane. It is a type of passive transport because during this transport ATP or energy is not used. The transport takes place along the concentration gradient. During osmosis (Endo/Exosmosis) solvent will always flow in that direction where solute concentration is high and water concentration is low.
Differences Between Exosmosis and Endosmosis
| Site of Difference | Exosmosis | Endosmosis |
|---|---|---|
| Solvent movement direction | The solvent flows out of the cells. | The solvent moves inside the cells. |
| Solute Concentration | Solute concentration is high in the medium outside the cell. | Solute concentration is low in the medium surrounding the cell. |
| Water potential | High in the cytosol. | High in the medium solvent outside the cell. |
| The fate of the cells | Cells shrink with water loss. | Cell swell up or turgid. |
| Examples | Water transport from the root hair to the cortical cells of the root. | Water transport from the root to the xylem vessels of the plant. |
**So, the correct answer is (B) Hypotonic solution **
Note: The processes of osmosis and diffusion play a major role in cell communication. It establishes a transport system across cells, for the distribution of-
Nutrients
Oxygen
Elimination of toxins
In 1748, J. A. Nollet first documented the observation of osmosis. The term was given by R. J. H. Dutrochet. The process of osmosis can be defined as the movement of a solvent across a semipermeable membrane to a region of higher concentration of solute and a lower concentration of solvent from a region of higher concentration of solvent and a lower concentration of solute.
There are two different kinds of osmosis:
Endosmosis
Exosmosis
