Question
Question: End product (p) of the above reaction is:  of the above reaction is:
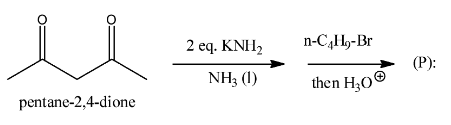
(A) 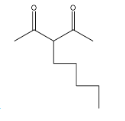
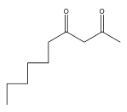
(B)
(C) 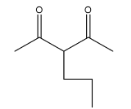
(D)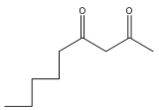
Solution
pentane-2,4-dione has two types of acidic hydrogen, one type of hydrogen lies in between two carbonyl groups, and the second type of acid hydrogen are with methyl groups. The loss of acidic hydrogens is going to depend on which type of base we are going to use.
Complete step by step solution:
In the given reaction a strong base called potassium amide is used in presence of liquid ammonia.
Then the base abstracts two acidic hydrogen from the reactant.
One acidic hydrogen is from −CH2− carbon and second hydrogen is from −CH3group and forms corresponding potassium derivative in the first step.
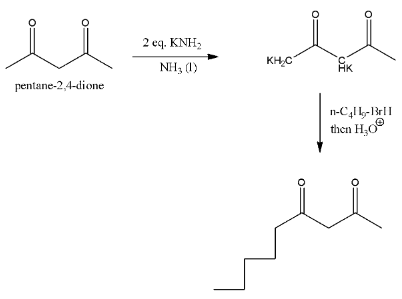
In the second step the formed potassium derivative reacts with alkyl halide (n-butyl bromide) and then hydrolysis forms a product substituted at less number of hydrogens.
Coming to given options,
option A
, it is not going to form because the other reactant is n-butyl bromide, it has four carbons, but in the answer A, there are five carbons there and that too they are attached at a wrong position.
Coming to option B, it is not going to form because the other reactant is n-butyl bromide, it has four carbons, but in answer B, there are five carbons.
Coming to option C, it is not going to form because the other reactant is n-butyl bromide, it has four carbons, but in answer C, there are three carbons and that too they are attached at a wrong position.
Coming to option D, it is correct because n-butyl bromide is attached at the correct position with a proper number of carbon atoms.
So, the correct option is D.
Note: Don’t be confused with the terms alkyl bromide and aryl bromide.
Alkyl bromide-it is a straight chain not containing any aryl (benzene groups) ring.
Aryl bromide- it contains an aryl (benzene group) ring.
