Question
Question: Elements X and Y belong to group 1 and \(17\) respectively of the periodic table. 1\. What will be...
Elements X and Y belong to group 1 and 17 respectively of the periodic table.
1. What will be the nature of the bond in the compound XY?
2. Give one property of XY.
3. Draw the electron dot cross structure of XY.
Solution
Periodic table is a chemical table in which all the elements (metals, nonmetals and metalloids) are arranged in periods (horizontal rows) and groups (vertical columns) according to their properties and atomic numbers. On moving down a group or moving from left to right in a period, there are some trends of chemical properties according to which we will answer this question.
Complete answer:
We know that in a periodic table all the elements (metals, nonmetals and metalloids) are arranged in periods (horizontal rows) and groups (vertical columns) according to their properties and atomic numbers.
1. Group 1 elements are known as alkali metals. They are electropositive in nature and can donate an electron to attain stability. Group 17 elements are halogens which are electronegative in nature and they are considered as non-metals.
So, when non-metal and metal combine to form a compound, there are electrostatic interactions and an ionic bond is formed between them. Hence, the nature of the bond in the compound XY is ionic.
2. XY is a neutral salt.
3. Electron dot structure of XY is given as:
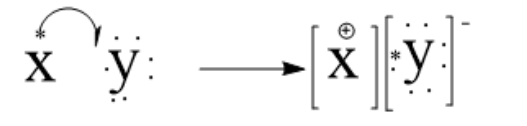
Note:
We have come to this conclusion because of the chemical properties of various elements of the periodic table. Based on these chemical properties and group numbers, we illustrated the electron dot structure of the compound. Group one also contains hydrogen atoms and if the hydrogen atom reacts with the halogens then an acid is formed.
