Question
Question: Element A and B do not form alloy because A) Both elements have similar crystal structures B) Ra...
Element A and B do not form alloy because
A) Both elements have similar crystal structures
B) Radius of A is 115pm while radius of B is 187pm
C) Both are the members of same group
D) Both have similar electronic configuration in valence shell
Solution
Hint Alloys have useful properties than metals; these are formed by melting a metal and dissolving other elements in it. The elements to be dissolved together must have almost the same radius. Their radius should not show variation with respect to one another. Bronze was the first alloy to be discovered.
Complete answer: Radius of element A is 115pm and radius of element B is 187pm. The difference in radius of both the elements is 72pm which is quite large. Both these elements when dissolved together will not suit the required conditions for the formation of alloy. Thus element A and element B will not form an alloy if their radii are different. So, the correct choice is (B).
Alloys are used because they enhance mechanical or chemical properties. Alloying elements can be added to a metal to increase no. of properties which includes hardness, strength, corrosion resistance, machinability, etc.
Additional Information:
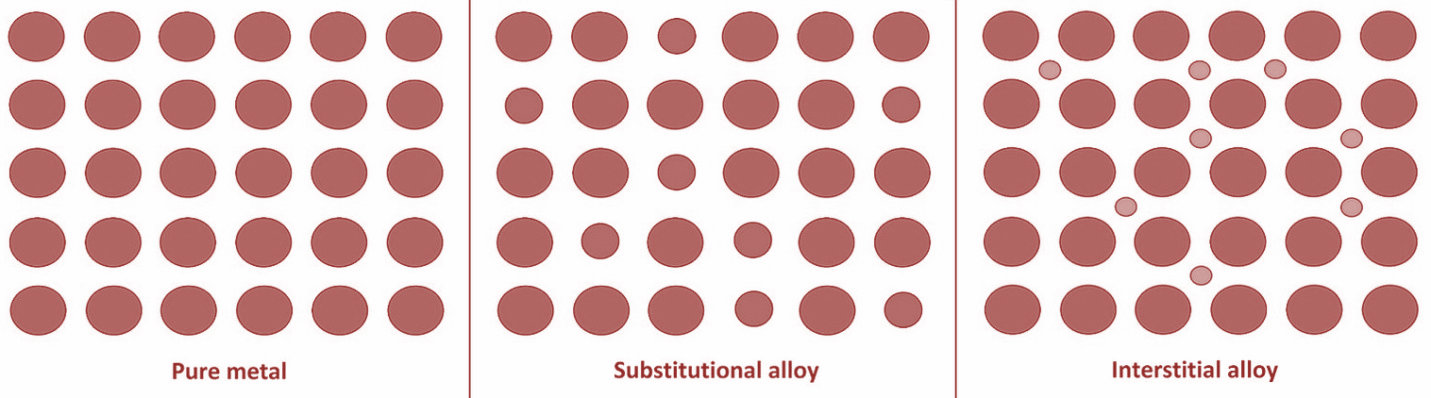
There are two types of alloys- Substitutional alloys and Interstitial alloys. In substitution alloys, the atoms of the original metal are replaced with atoms that have roughly the same size from another material ex- Brass, in which some of the copper atoms are substituted with either tin or zinc atoms. The different amount and strength of covalent bonds can change depending on the different specific metals involved and how they are mixed. The covalent bonding is responsible for the crystal structure as well as the melting point and various other physical properties.
Interstitial alloy is formed when an atom of sufficiently small radius sits in an interstitial hole in metal lattice ex- hydrogen, boron, carbon.
A metal alloy is formed by combining it with one or more other elements. The most common alloying process is performed by heating the base metal beyond its melting point and then dissolving the solutes into the molten liquid. For example, in its liquid state, titanium is a very strong solvent capable of dissolving most metals and elements. In addition, it readily absorbs gases like oxygen and burns in the presence of nitrogen.
Stainless Steel is an example of a combination of Interstitial and Substitutional alloys, because the carbon atoms fit into the interstices, but some of the iron atoms are substituted by nickel and chromium atoms.
Note: Tensile strength of alloy is more than its constituent elements. Metals melt at very high temperatures. Metals are quite susceptible to chemical and weather attacks. Alloying can be used to change the color of the base metal. So alloying the elements is quite useful and has many advantages over the constituent metals. One can study about the different types of alloys and the elements from which they are formed.
