Question
Question: Electrophilic addition reaction is not shown by: (a) 2 methyl propene and bromine (b) Acetylene ...
Electrophilic addition reaction is not shown by:
(a) 2 methyl propene and bromine
(b) Acetylene and HOCl
(c) Propyne and MeMgBr
(d) Ethene and dilute sulphuric acid
Solution
An addition reaction, where a pi bond is broken and new sigma bonds are formed is called electrophilic addition reaction in organic chemistry. The reactant must contain a double bond or triple bond.
Complete step by step solution: The driving force of an electrophilic addition reaction is an electrophile (X+) that can form covalent bonds with an electron-rich unsaturated bond. Mechanism of the reaction is that the positive charge on the electrophile is transferred to the carbon-carbon bond, forming a carbocation during the formation of the C-X bond. Then this positively charged intermediate combines with electron rich (usually an anion) to form a second covalent bond.
Let us consider each option given in the question.
-2 methyl propene and bromine react to give an additional product. Liquid bromine can react with alkenes to give additional products. The double bond is replaced with a sigma bond between the methylene group and one of the bromine.
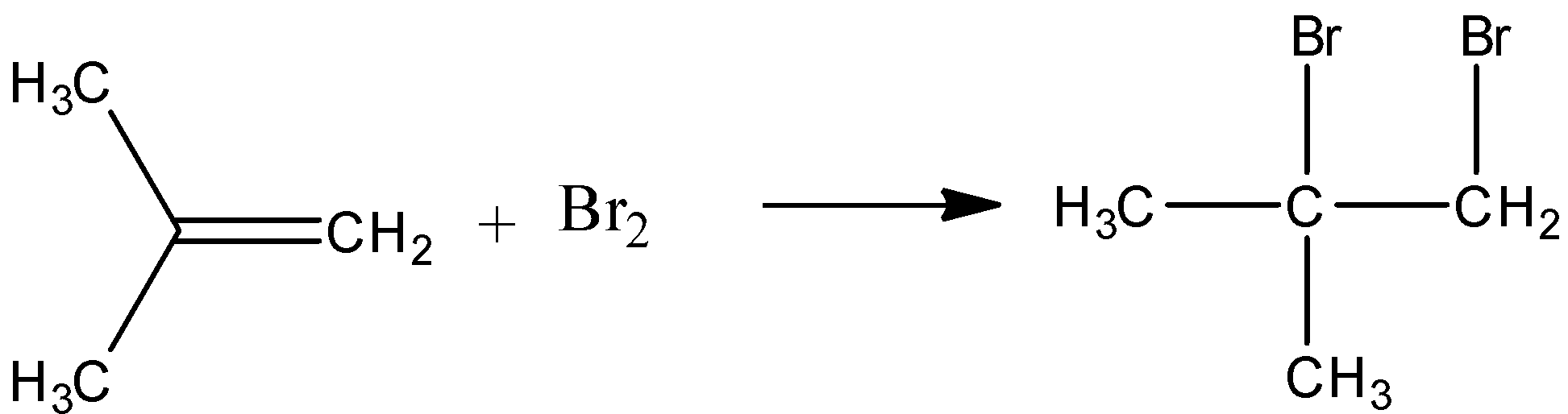
-When acetylene reacts with HOCl dichloro acetaldehyde is formed. Here oxidation addition of acetylene to aldehyde occurs. The reaction is as follows.
HC≡CH+2HOCl→HCCl2−C(OH)2H−H2OHCCl2−CHO
-In case of reaction between propyne and MeMgBr, the acidic proton of propyne is abstracted by CH3− group of the Grignard reagent methyl magnesium bromide. Thus, here additional products are not formed.
-When ethene reacts with concentrated sulphuric acid, it produces alkyl hydrogen sulfates. This is also an electrophilic addition product.
CH2=CH2+H2SO4→CH3CH2OSO2OH
Thus, we can conclude that propyne and MeMgBr does not undergo electrophilic addition reaction and the correct option is (c).
Note: Only when there is a double or triple bond is present electrophilic addition occurs. Here an electrophile attacks the unsaturated part of the compound and forms sigma bonds.
