Question
Question: Which of the following represent correct stability order:...
Which of the following represent correct stability order:
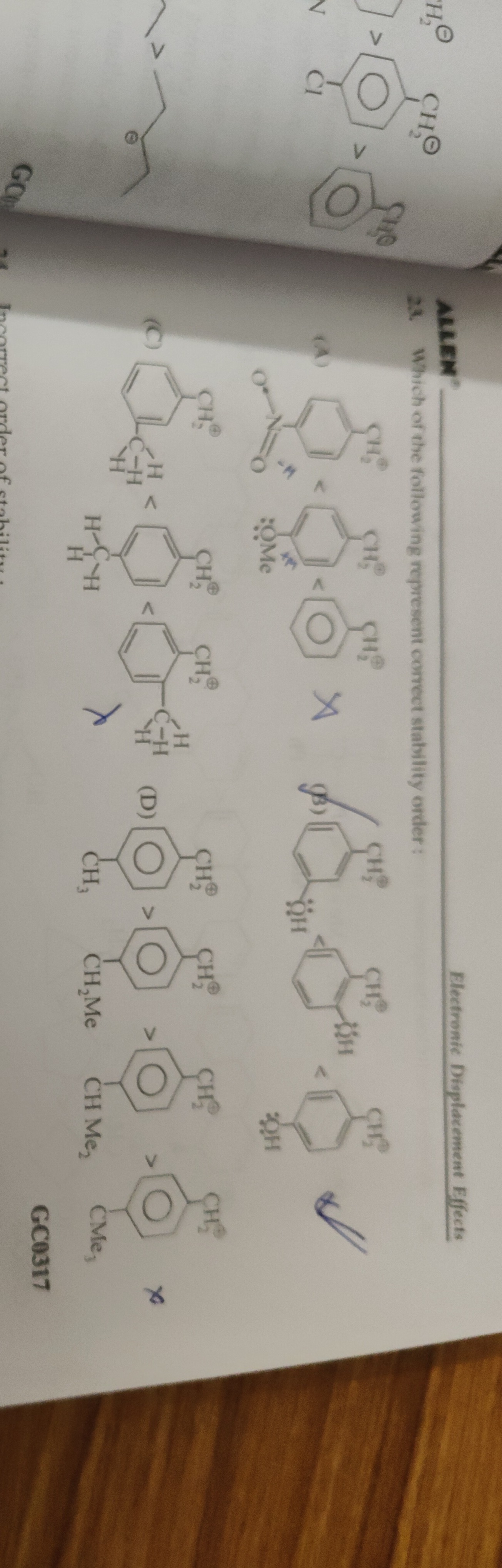
p-nitrobenzyl carbocation < p-chlorobenzyl carbocation < benzyl carbocation < p-methylbenzyl carbocation
p-hydroxybenzyl carbocation < p-methoxybenzyl carbocation < benzyl carbocation < p-methylbenzyl carbocation
p-methylbenzyl carbocation < benzyl carbocation < p-chlorobenzyl carbocation < p-nitrobenzyl carbocation
p-isopropylbenzyl carbocation < p-isopropylbenzyl carbocation < p-t-butylbenzyl carbocation < p-methylbenzyl carbocation
p-nitrobenzyl carbocation < p-chlorobenzyl carbocation < benzyl carbocation < p-methylbenzyl carbocation
Solution
The stability of carbocations is influenced by electronic effects. Electron-donating groups stabilize carbocations, while electron-withdrawing groups destabilize them.
- p-nitrobenzyl carbocation: The nitro group (-NO2) is a strong electron-withdrawing group (EWG) via inductive (-I) and resonance (-M) effects, significantly destabilizing the carbocation.
- p-chlorobenzyl carbocation: The chlorine atom (-Cl) is an EWG due to its stronger inductive effect (-I) compared to its resonance donating effect (+M).
- Benzyl carbocation: Stabilized by resonance delocalization of the positive charge into the benzene ring.
- p-methylbenzyl carbocation: The methyl group (-CH3) is an electron-donating group (EDG) via inductive (+I) and hyperconjugation effects, providing stabilization.
The order of stability based on these effects is: Strong EWG (-NO2) < Weak EWG (-Cl) < No significant effect (H in benzyl) < EDG (-CH3). Therefore, the correct stability order is: p-nitrobenzyl carbocation < p-chlorobenzyl carbocation < benzyl carbocation < p-methylbenzyl carbocation.
