Question
Question: Electromagnetic radiation of wavelength 663 nm is just sufficient to ionize the atom of metal A. The...
Electromagnetic radiation of wavelength 663 nm is just sufficient to ionize the atom of metal A. The ionization energy of metal A in kJ mol−1 is __. (Rounded off to the nearest integer) [h = 6.63 × 10−34 J-s, c = 3.00 × 108 ms−1, NA = 6.02 × 1023 mol−1]
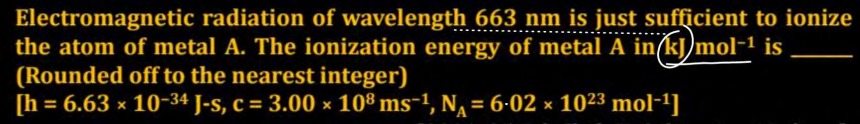
Answer
181
Explanation
Solution
The energy of a photon is given by E=λhc. The ionization energy for one mole of atoms is I.E.=λhcNA. Substituting the given values: I.E.=663×10−9 m(6.63×10−34 J-s)×(3.00×108 ms−1)×(6.02×1023 mol−1) I.E.≈180600 J mol−1 Converting to kJ mol−1: I.E.≈180.6 kJ mol−1 Rounded to the nearest integer, the ionization energy is 181 kJ mol−1.
