Question
Question: 100 L aqueous solution of KBr is electrolysed by using current of 9.65 A for 10 second using platinu...
100 L aqueous solution of KBr is electrolysed by using current of 9.65 A for 10 second using platinum electrodes (assume 100% current efficiency).
How many of the following species can be produced during electrolysis of KBr using Pt electrodes?
H₂, Br₂, K, KOH, O₂
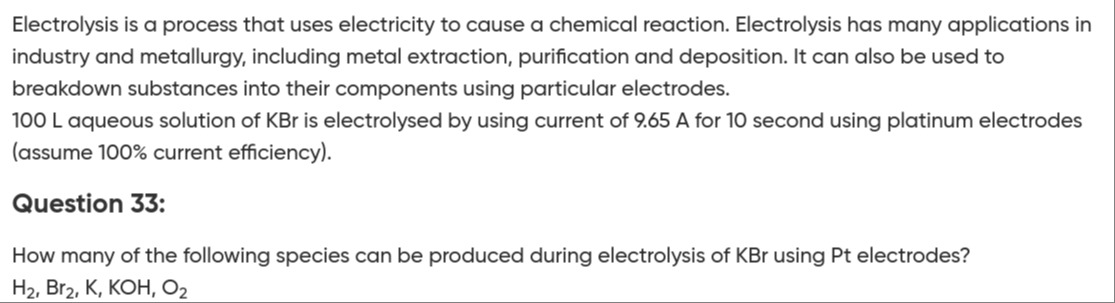
3
Solution
Electrolysis of an aqueous solution of KBr involves competing reactions at the anode (oxidation) and cathode (reduction). Platinum electrodes are inert, meaning they do not participate in the chemical reactions themselves.
The species present in the aqueous KBr solution are K⁺(aq), Br⁻(aq), and H₂O(l).
At the Cathode (Reduction): Possible reduction reactions are:
-
Reduction of K⁺ ions: K⁺(aq) + e⁻ → K(s) Standard reduction potential, E° = -2.92 V
-
Reduction of water: 2H₂O(l) + 2e⁻ → H₂(g) + 2OH⁻(aq) Standard reduction potential, E° = -0.83 V (at pH 7)
Comparing the standard reduction potentials, water has a significantly less negative (more positive) reduction potential (-0.83 V) than K⁺ ions (-2.92 V). Therefore, water will be preferentially reduced at the cathode. Cathode Reaction: 2H2O(l)+2e−→H2(g)+2OH−(aq) Species produced at the cathode: H₂ gas and OH⁻ ions. The OH⁻ ions will combine with the spectator K⁺ ions in the solution to form potassium hydroxide (KOH), making the solution alkaline.
At the Anode (Oxidation): Possible oxidation reactions are:
-
Oxidation of Br⁻ ions: 2Br−(aq)→Br2(l)+2e− Standard oxidation potential = -E°(Br₂/Br⁻) = -1.09 V
-
Oxidation of water: 2H2O(l)→O2(g)+4H+(aq)+4e− Standard oxidation potential = -E°(O₂/H₂O) = -1.23 V
Comparing the standard oxidation potentials, Br⁻ ions have a less negative (more positive) oxidation potential (-1.09 V) than water (-1.23 V). Therefore, Br⁻ ions will be preferentially oxidized at the anode. Anode Reaction: 2Br−(aq)→Br2(l)+2e− Species produced at the anode: Br₂ liquid (which can appear as a reddish-brown liquid or vapor).
Summary of Species Produced: Based on the preferred reactions:
- At the cathode: H₂ gas and OH⁻ ions (which form KOH in solution).
- At the anode: Br₂ liquid.
Therefore, the species produced during the electrolysis of aqueous KBr using Pt electrodes are H₂, Br₂, and KOH.
Let's check the given list of species:
- H₂: Yes, produced at the cathode.
- Br₂: Yes, produced at the anode.
- K: No, K⁺ ions are not reduced to K metal.
- KOH: Yes, formed in the solution from K⁺ and OH⁻ ions.
- O₂: No, Br⁻ ions are oxidized in preference to water.
Thus, three species (H₂, Br₂, KOH) can be produced.
