Question
Question: During the manufacture of cast iron, the slag \[\left( {CaSi{O_3}} \right)\] is formed in: A.Zone ...
During the manufacture of cast iron, the slag (CaSiO3) is formed in:
A.Zone of fusion only
B.Zone of reduction and Zone of fusion
C.Zone of heat absorption
D.Zone of reduction only
Solution
The solution of the problem is revolving about the Blast furnace for the manufacture of iron. The temperature in a blast furnace varies from 16000C to 2500C from bottom to top. The temperature increases from top to bottom. Due to this variation in temperature range, four different zones are formed where different chemical changes occur.
Complete step by step answer:
The shape of the blast furnace is a huge cylindrical furnace almost 30m in height and 5m in diameter. The process is as follows the blast furnace is heated with a blast of air and due to the variation in temperature four zones are created where different chemical changes occur. These four changes are as follows:
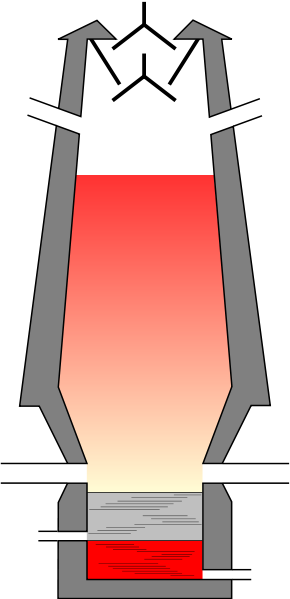
First zone is zone of fusion. In this zone temperature remains between (12000−15000C). In this zone the iron gets fused and appear in hearth while the slag floats.
Second zone is the zone of combustion. Zone of combustion is present in the lower part of the blast furnace and in this zone the temperature range is about (15000−16000C). As this zone is of combustion, carbon gets burned in presence of air and produces carbon dioxide and large amounts of heat.
C+O2→CO2+97.0Kcal
After that carbon dioxide produced in the zone rises up and reacts with hot coke (C) and produces carbon monoxide.
CO2+C15000C2CO
Third zone is the zone of reduction. This zone is the upper most part of the blast furnace where temperature range varies from (2500−7000C). In this zone ore is reduced to iron as follows:
3Fe2O3+CO300−4000C2Fe3O4+CO2
Fe3O4+CO500−6000C3FeO+CO2
Fe3O4+4CO→3Fe+4CO2
Fourth Zone is the zone of heat absorption. This zone is also called the middle zone or zone of slag formation of blast furnaces and the temperature range in this zone is (8000−10000C). The slag is formed as follows:
CaO+SiO3→CaSiO3
Final Result: From above four zones we can easily conclude that the slag (CaSiO3) is formed in a zone of heat absorption.
Therefore, the correct option is (C).
Note: The most important thing to note here is that Zone of heat absorption is also called lower zone of reduction. So there may be an option given in the question that slag (CaSiO3) is formed in the zone of heat of absorption and zone of reduction.
