Question
Question: During change of \({{\text{O}}_{2}}\) to \({{\text{O}}_{2}}^{-}\) ion, the electron adds on which on...
During change of O2 to O2− ion, the electron adds on which one of the following orbitals?
A. π orbitalB. σ∗orbitalC. σ orbitalD. π∗ orbital
Solution
The question deals with diatomic molecule which are O2 and O2−. Diatomic molecules and their configuration comes under Molecular orbital theory. It is a method that is used to describe the electronic structure of diatomic molecules using quantum mechanics.
Complete Solution:
The addition of electrons in which an orbital cannot be explained without knowing what is molecular orbital theory and its general configuration. Let us first see the definition; This theory uses a linear combination of atomic orbitals which represents molecular orbitals that result from bonds between atomic orbitals. It is categorized into three types: bonding, antibonding and non-bonding.
Oxygen molecule or O2- The electronic configuration of oxygen (Z = 8) in its ground state is 1s22s22p4. Oxygen atoms have 8 electrons thus; there are 16 electrons in O2 molecule. The electronic configuration of O2:
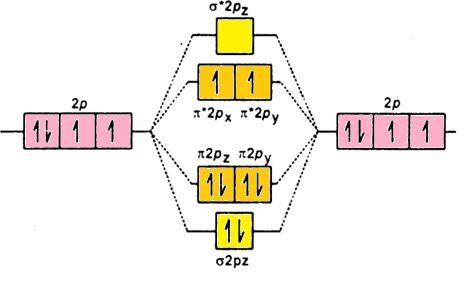
O2:σ1s2σ∗1s2σ2s2σ∗2s2σ2pz2π2px2π2py2π∗2px1π∗2py1; the electrons in molecular orbitals are filled according to Hund’s Rule and Aufbau rule.
- Hund’s Rule: Each orbital in a subshell is filled with one electron before its following orbital is filled again and all electrons which are singly filled in the orbitals have the same spin. See the difference between the configurations,
CORRECT− ↑ ↑↑ INCORRECT− ↑↓ ↑
- Aufbau Rule- It states that in the ground state of an atom; electrons fill orbitals of the lowest energy levels before occupying higher levels. Like 3d is filled before 4s. Its molecular orbital diagram is (p-orbital):
Let us see the electronic configuration ofO2− it has total ( 8 + 8 + 1) electrons in it that is total 17 electrons:
O2−: σ1s2σ∗1s2σ2s2σ∗2s2σ2pz2π2px2π2py2π∗2px2π∗2py1: addition of electron took place in the antibonding orbital which is π2px∗, the number of electrons has increased from 1 to 2 in that orbital. Its molecular orbital diagram is (p-orbital):
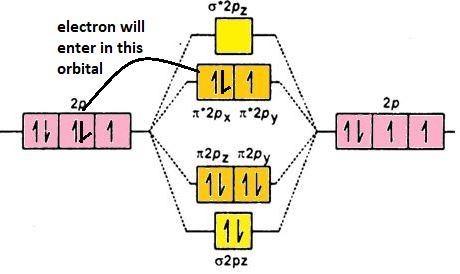
Thus, the correct answer to this question is the incoming electron added to π∗
So, the correct answer is “Option D”.
Note: The electronic configuration for elements having electrons greater than 14 electrons is different with the configuration of elements having electrons less than equal to 14. Let us see the electronic configuration:
(1) Greater than 14 electrons: σ1sσ∗1sσ2sσ∗2sσ2pzπ2pxπ2pyπ∗2pxπ∗2py
(2) Electrons less than equal to 14 electrons: σ1sσ∗1sσ2sσ∗2sπ2pxπ2pyπ∗2pxσ2pzπ∗2pyσ2pz∗
