Question
Question: Draw the tetrahedral dimer structure of \(AlC{{l}_{3}}\)...
Draw the tetrahedral dimer structure of AlCl3
Solution
Compounds having the formulaAlCl3(H2O)n (n = 0 or 6) are known as aluminium chlorides. They are made up of aluminum and chlorine atoms in a 1:3 ratio, with one form containing six hydration fluids. Both are white solids, although samples frequently contain iron(III) chloride, which gives them a yellow hue.
Complete answer:
Aluminium chloride is a chlorine-aluminum compound. The solid is covalently linked and has a low melting and boiling point. At 178 degrees Celsius, it reaches its pinnacle. Unlike more ionic halides like sodium chloride, moltenAlCl3conducts electricity weakly. It has a six-coordinate layer lattice in the solid state. The structure ofAlCl3is "YCl3," withAl3+ cubic tightly packed layered structure. WhenAlCl3is melted, it forms the dimerAl2Cl6, which may be vaporized. ThisAl2Cl6dimer dissociates into trigonal planarAlCl3at higher temperatures.
AlCl3 can form a dimer,Al2Cl6, because aluminium possesses empty d-orbitals that can accommodate an electron from the chlorine atom.AlCl3 is an electron deficient (octet incomplete) combination in Al3+, thus it acts as a Lewis acid, and Al completes it by taking an electron pair from the Cl− atom, as indicated in the picture.
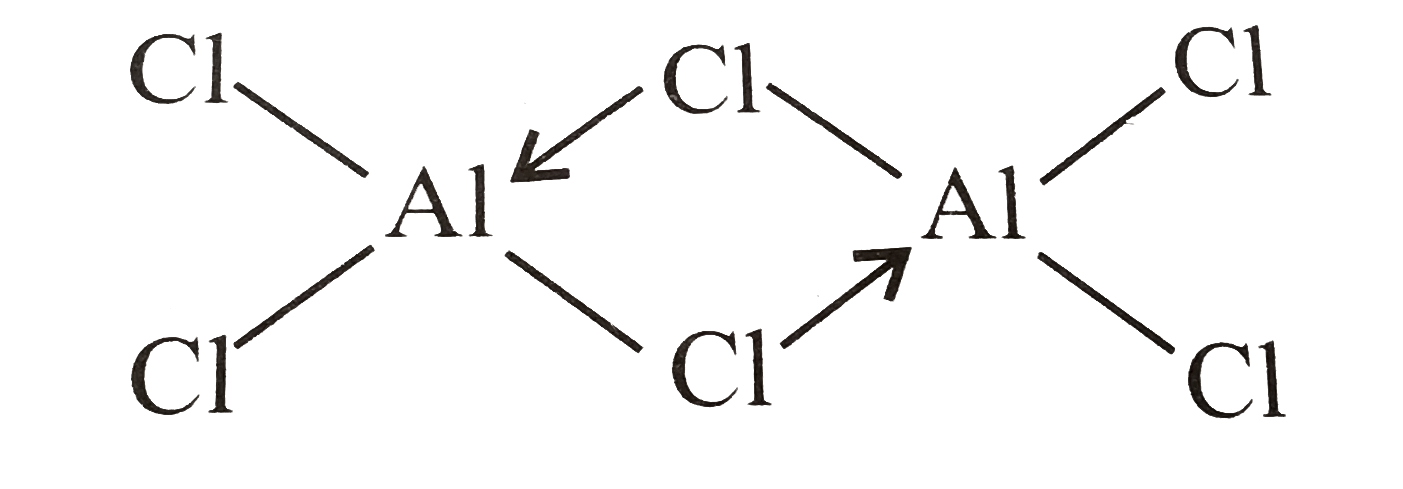
A dimer is an oligomer made up of two monomers linked by covalent or intermolecular interactions that might be strong or weak. When the two molecules are identical (e.g. A–A), the word homodimer is used, and when they are not (e.g. A–B), the term heterodimer is used. Dissociation is the polar opposite of dimerisation. Bjerrum pairs are named after Niels Bjerrum, who discovered that two oppositely charged ions may form dimers.
Note:
Aldehyde groups can be brought in or attached to aromatic series or rings using aluminum chloride. Consider the Gatterman-Koch reaction, in which the Lewis acid (aluminum chloride) is employed to extract a chloride ion from a species. It's also utilized in light molecular weight hydrocarbon polymerization and isomerization processes. Ethylbenzene synthesis and dodecylbenzene production for detergents are two common examples. Bis(arene) metal complexes can be made by mixing aluminum chloride with aluminum and arene.
