Question
Question: Draw the structures of the following molecules: A.\({{H}_{3}}P{{O}_{2}}\) B.\(Cl{{F}_{3}}\)...
Draw the structures of the following molecules:
A.H3PO2
B.ClF3
Solution
In this question, we have to draw the structures of H3PO2and ClF3. H3PO2 which is known as Hypophosphorous acid. It is a phosphorus oxyacid which is a strong reducing agent. It is also called phosphinic acid. ClF3is chlorine trifluoride which is formed by fluorination of chlorine.
Complete answer:
H3PO2- It is a colorless compound that is water-soluble. It’s a low-melting compound. It is also soluble in dioxane and alcohol. The more descriptive representation of it is HOP(O)H2. It has a monoprotic character. Hypophosphites are the name of the salts that are derived from this acid.
HOP(O)H2 occurs in the equilibrium with slight tautomer HP(OH)2, It is called hypophosphorous acid whereas the major tautomer is phosphonic acid.
Preparation-
The white phosphorus reacts with a hot aqueous solution of a hydroxide results in the formation of hypophosphite salts.
P4+OH−+4H2O→4H2PO2−+2H2
A strong non-oxidizing acid is reacted with the salt to give hypophosphorous acid.
H2PO2−+H+→H3PO2
H3PO2is used as a 50% aqueous solution. The evaporation of water cannot form anhydrous acid because the acid disproportionates to phosphorus and phosphine, it gets oxidized to phosphorous acid and phosphoric acid. The continuous extraction of aqueous solution with diethyl ether forms pure anhydrous hypophosphorous acid.
Structure of H3PO2 is :
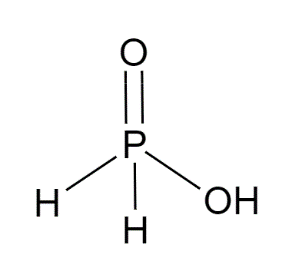
The molecular shape of H3PO2is pseudo-tetrahedral.
ClF3- In 1930, Ruff and Krug prepared it by fluorination of chlorine, It also gave ClF, and Distillation was used to separate the mixture.
Preparation-
3F2+Cl2→2ClF3
Structure of ClF3 is :

The molecular geometry of ClF3 is T-shaped.
The structure was predicted by VSEPR theory.
Note:
Uses of H3PO2 are- It is utilized as a salt (Sodium hypophosphite). It is also used in the electroless plating of Nickel.
Uses of ClF3are- It helps in cleaning of the chemical vapor deposition chambers in the semiconductor industry. It can be used as a storable oxidizer in rocket propellant systems.
