Question
Question: Draw the structures of the following molecules: (A) \(Br{F_3}\) (B) \({(HP{O_3})_3}\) (C) \(Xe...
Draw the structures of the following molecules:
(A) BrF3
(B) (HPO3)3
(C) XeF4
Solution
Molecules are generally held together by covalent bonds or shared pairs of electrons. These bonds are bidirectional, which means the atoms adopt specific positions relative to one another in order to maximize the bond strengths. So, each molecule will have a definite structure or spatial distribution of atoms.
Complete answer:
a) BrF3:
The electronic configuration of bromine is 1s22s22p63s23p63d104s24p5. BrF3 has seven electrons in its outermost shell and after bond formation it will have two lone pairs and three covalent bonds of bromine and fluorine. As the electron pair is equal to five the hybridisation is sp3d. The molecular geometry is trigonal bipyramidal and the bond angle is 86.2∘. The structure of BrF3 is given below:
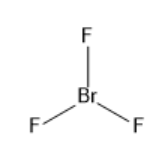
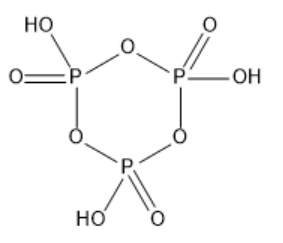
b) (HPO3)3:
The compound is called trimetaphosphoric acid. It is made up of three Meta phosphoric acid arranged in a cyclic structure. The structure of (HPO3)3 is given below:
c)XeF4:
The valence shell of xenon has six electrons in the 5p orbital and two electrons in the 5s orbital. In the formation ofXeF4, two electrons of the 5p orbital move to fill the vacant 5d orbitals. So, there will be four unpaired electrons. Hybridization will besp3d2. The structure of xenon tetrafluoride is given below:
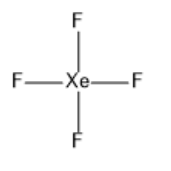
Note:
The concept of mixing of atomic orbitals to form new hybrid orbitals having different energies, shapes, etc. than the component atomic orbitals, suitable for the pairing of electrons to form chemical bonds in valence bond theory is hybridization.
