Question
Question: Draw the structures of the following: a. \({\rm{Xe}}{{\rm{F}}_4}\) b. \({\rm{Br}}{{\rm{F}}_5}\)...
Draw the structures of the following:
a. XeF4
b. BrF5
Solution
The total number of bond pairs and lone pairs around the central atom can be used to deduce the geometry of the molecules as per VSEPR theory.
Complete answer
a. We know that VSEPR theory is based on the repulsion between valence shell electrons. These electrons can be bonded or non-bonded thus it is the total number of valence shell electrons on the central atom in a molecule that would decide the structure of the molecule. This theory has given simple stable geometries for different types of molecules. For example, a molecule in which the central atom has only two bond pairs, will have a linear structure but in case it has two bond pairs and a lone pair, the structure would be bent. Now, let’s have a look at the given molecules one by one:
The first molecule given to us is XeF4. Here, xenon has a total eight valence electrons out of which four have been used in the bond formation with fluorine. So, we can say that there are four bond pairs around xenon and two lone pairs accounting for the remaining four valence electrons. Now, as per VSEPR theory, the structure for XeF4 would be square planar that can be drawn as follows:
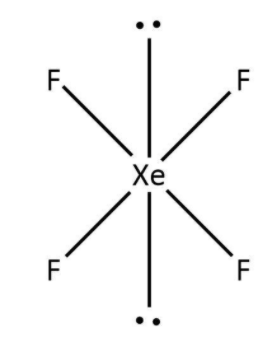
b. The second molecule given to us is BrF5. Here, bromine has a total seven valence electrons out of which five have been used in the bond formation with fluorine. So, we can say that there are five bond pairs around bromine and one lone pair accounting for the remaining two valence electrons. Now, as per VSEPR theory, the structure for BrF5 would be square pyramid that can be drawn as follows:
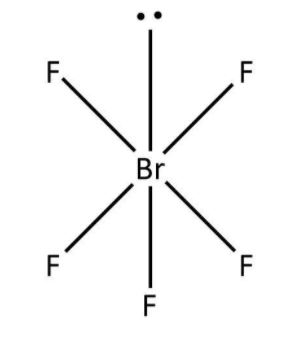
Note:
The arrangement of electron pairs in both the molecules is octahedral as there are a total six pairs but the shape is different as the number of bond pairs and lone pairs are not the same.
