Question
Question: Draw the structures of (i) \({\text{Xe}}{{\text{F}}_{\text{2}}}\) and (ii) \({\text{Br}}{{\tex...
Draw the structures of
(i) XeF2 and
(ii) BrF3
Solution
A Lewis Electron Dot structure or a Lewis Diagram or a Lewis structure is a representation of the valence shell electrons of an atom by using dots around the symbol of the element.
Complete answer:
(i) XeF2:The valence shell of the xenon has a vacant 5d orbital and one 5d orbitals along with the 5s and 5p orbitals hybridize together to form five sp3dhybrid orbitals for the central xenon atom. These hybrid orbital on the central atom three lone pairs and two bond pairs that now form covalent bonding with by sharing electrons with the fluorine atoms that can be represented as follows:
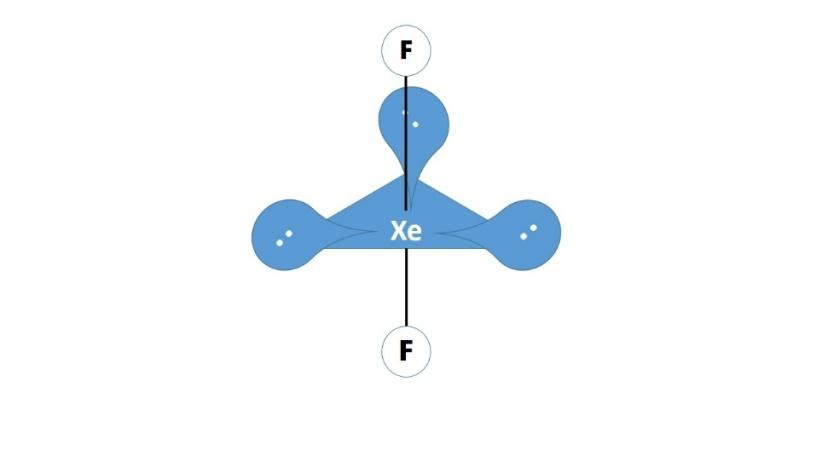
(ii) BrF3: The valence shell of the bromine has a vacant 4d orbital and one 5d orbitals along with the 5s and 5p orbitals hybridize together to form five sp3d hybrid orbitals for the central bromine atom. These hybrid orbital on the central atom two lone pairs and three bond pairs that now form covalent bonding with by sharing electrons with the fluorine atoms that can be represented as follows:
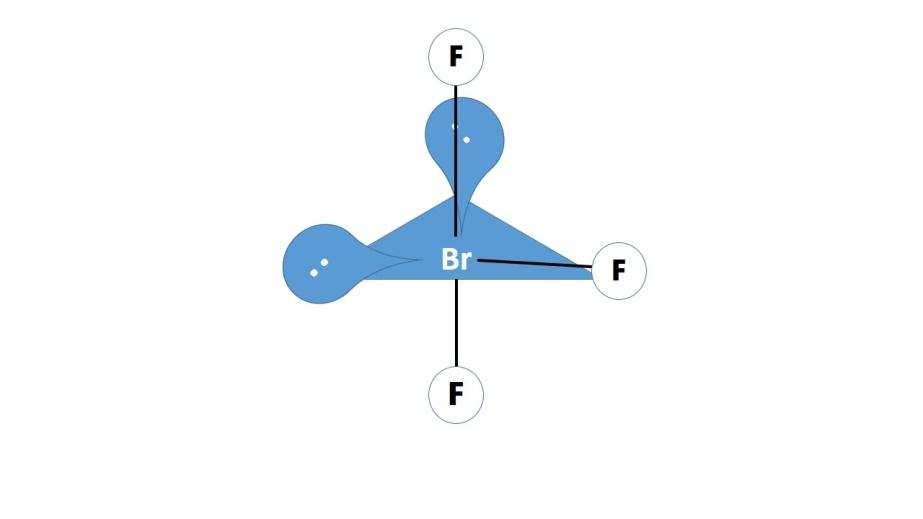
Note:
In the electronic structure of xenon fluoride, there are eight electrons in the outer shell of the xenon atom. There are seven electrons in the valence shell of the fluorine atoms. The electronic configuration of xenon atom is as follows: 4d105s25p6. In the electronic structure of bromine trifluoride, there are seven electrons in the outer shell of the bromine atom and seven electrons in the valence shell of the fluorine atoms. The electronic configuration of xenon atom is as follows: 4s23d104p5.
