Question
Question: Draw the structures of following molecules (i) \({{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\) ...
Draw the structures of following molecules
(i) N2O5 (ii) XeF2
Solution
To draw the structure of a molecule write the Lewis structure. Lewis structure shows the electrons around the atoms of a molecule.
Complete answer:
Write the Lewis structure as follows:
Write the basic structure. Write the central atom around which writes all atoms of the molecule. The least electronegative atom is the central atom. Count total valence electrons. Two electrons are used in the formation of a bond. Count the total electron used in bond formation. Subtracts the electrons used in bond formation from the total valence electrons.
Arrange the remaining electrons around each atom to complete the octet.
(i) The Lewis structure of N2O5 is as follows:
Total valence electrons are as follows:
=(5×2)+(6×5)
=40 16
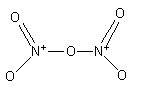
The total electron used in the bonds are.
The remaining valence electrons are,
=40−16
=24
Arrange the 24 electrons to complete the octet of each atom.
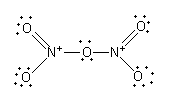
The nitrogen-oxygen double bond is not fixed. The nitrogen can form a double bond with the other two oxygen atoms.
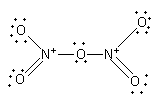
N2O5
(ii) The Lewis structure of is as follows:
Total valence electrons are as follows:
=(8×1)+(7×2)
=22
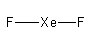
The total electron used in the bonds are.
The remaining valence electrons are,
=22−4
=18
Arrange the 18 electrons to complete the octet of each atom.
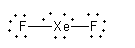
So, the structure of XeF2is linear.
Note: Each atom requires eight electrons to complete an octet except hydrogen. The hydrogen requires two electrons to complete the octet. Nitrogen pentoxide has two coordination bonds. In a covalent bond both the bonded atoms donate an electron but when only one atom donates two electrons for the formation of a bond then the bond is known as a covalent bond.
