Question
Question: -Draw the structure which shows synergic bonding interaction in a carbonyl complex?...
-Draw the structure which shows synergic bonding interaction in a carbonyl complex?
Solution
Synergic bonding involves transference of electrons from ligands to metal and the transference of electrons from filled metal orbitals to anti-bonding orbitals of ligands.
Complete step by step answer:
Synergic bonding is a bond between a ligand and a metal where a carbonyl group acts as a ligand. The carbonyl group CO is a pi acid ligand where pi-acid ligands are those which have a lone pair of electrons to donate a metal atom and an empty anti bonding molecular orbital to back bond with the d-orbital electrons of metal atom. Synergic bonding is represented as,
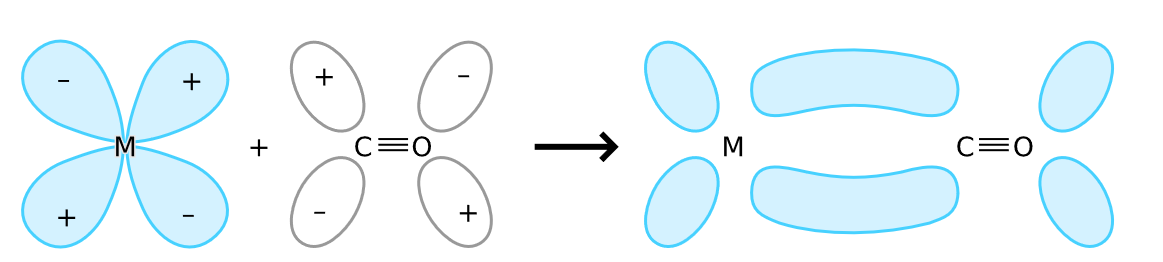
Carbonyl donates a lone pair of electrons to metal to form a M−Cσ bond. Filled d orbital of metal donates lone pair of electrons to vacant anti bonding π orbital of CO to form M−Cπ bond.
Additional Information:- Synergic bonding is a self-strengthening bond. In synergic bonding, the electrons are partially transferred from a d-orbital of the metal to anti bonding molecular orbitals of CO. This electron transfer strengthens the metal-C bond and weakens the C−O bond. The strengthening of the M−CO bond is reflected in the increase of the vibrational frequencies for the M−C bond. The M−CO bond length is shortened. The weakening of the C−O bond is indicated by a decrease in the wave number.
Note:
Synergic bonding is also known as pi-back bonding. It is usually used in organometallic chemistry when there is a transition metal centre and a good pi acceptor ligands like CO. Many ligands other than CO are strong backbonders.
