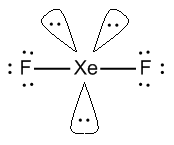Question
Question: Draw the structure of \[Xe{F_2}\] molecule. Write the outer electronic configuration of Cr atom (z...
Draw the structure of XeF2 molecule. Write the outer electronic configuration of Cr atom
(z=24).
Solution
Molecular structure depends on the various repulsive effects between lone pairs and bond pairs. Molecular theory and valence shell electrons pair repulsion theory are used to determine the structure of the molecule. So we will try to figure out the shape of a given molecule with the help of these theories. Electronic configuration of a molecule is written by writing the total numbers of electrons in every orbital. Aufbau principle is used to fill the electrons in every orbital.
Complete step by step answer:
The first step in predicting the shape of the molecule is Lewis dot structure. Although the Lewis dot structure doesn't determine the shape, it can only predict the shape of the molecules. The next theory that was prescribed to determine the shape of the molecule is Valence shell electron pair repulsion theory. It states that electron pairs repel each other whether or not they are in lone pair or bond pair.
Thus to determine the exact shape of the molecule the location of the nuclei and electrons should be known. Next theory which comes into account was molecular orbital theory which gives the total information about the number of lone pairs, number of bond pairs, and angle.
- XeF2 or xenon difluoride, In XeF2, Xe has 8 valence electrons out of which two electrons are shared with 2 fluorine atoms.
- It has 2 sigma bonds and 3 lone pairs of electrons. In which the axial position is covered by fluorine atoms.
- XeF2 has a linear structure represented by F-Xe-F.
- Configuration of Cr atom is 1s22s22p63s23p64s13d5
- Therefore we can conclude that XeF2 has linear shape and the configuration of Cr atom is 1s22s22p63s23p64s13d5
Note:
Every species wants to attain stability because having higher energy will make the compound unstable, and half filled or completely filled orbitals are more stable than incomplete ones. So to attain the half filled configuration Cr has this configuration.