Question
Question: Draw the structure of the silicate anion in: (a) \({K_4}Si{O_4}\) (b) \(A{g_{10}}S{i_4}{O_{13}}\...
Draw the structure of the silicate anion in:
(a) K4SiO4
(b) Ag10Si4O13
Solution
Silicate is the member of a group of anions which consists of silicon and oxygen. The general formula of silicates is [SiO4−x(4−2x)−]n where x can be 0 or greater than 0 and less than 2. The lowest form of silicates is SiO44−.
Complete step-by-step answer:
In the compound K4SiO4 the anion part is SiO44−. It is called orthosilicate. Orthosilicate is sometimes called silicon tetroxide because there is one Si and four oxygen surrounds it. Silicates of potassium are manufactured in ordinary furnaces of glass. The uses of potassium orthosilicate are that it is used as adhesive and sealant chemical. It can also be used as agricultural chemical, plating and surface treating agents. For industrial and commercial use application, it is a hard surface cleaner. The structure of orthosilicate or SiO44− is as follows,
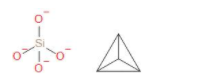
In the compound Ag10Si4O13 the anion part is Si4O1310−. Ag10Si4O13 exhibits excellent photocatalytic activity towards degradation of organic molecules. The structure of Si4O1310− is as follows,
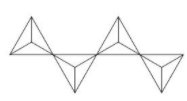
Here in this compound, oxygen attached is shared between two Si atoms.
Note: Solid silicates are usually stable and have been characterized well. Silicates with alkali cations are partially soluble in water. Silicate can be natural and artificial and they can be used for artistic activity. The most abundant mineral groups on earth are silicates. They have many technological uses too. The quartz crystals used in watches and radios are also silicates which can produce rhythmic high frequency vibration. To protect from extreme temperatures and outer atmosphere, silicate ceramic tiles possessing thermal properties are used on space shuttles.
