Question
Question: Draw the structure of the following molecules: (i) \(XeO{F_4}\) (ii) \({H_3}P{O_3}\)...
Draw the structure of the following molecules:
(i) XeOF4 (ii) H3PO3
Solution
First try and find out the valence shell electronic configuration of the central atom. Then try to give structure to the compound in a way that all the atoms forming the bonds complete their octet.
Complete answer:
In order to draw the structure of the given molecules, we need to know the bonding between the atoms. We will see their structure one by one.
i) XeOF4:
- We know that Xe (Xenon) is a noble gas having an atomic number of 54. However, it makes complexes.
- The valence shell electronic configuration of Xe is [Kr]4d105s25p6. So, there are 8 electrons in the valence shell of Xe. There are 6 and 7 electrons in the valence shell of oxygen and fluorine respectively.
- So, Xenon should make four single bonds with four fluorine atoms in order to satisfy the octet of fluorine. Xenon will make a double bond with oxygen as it will complete the octet of oxygen atoms. There will be one lone pair on the Xenon atom.
- Thus, we can say that the hybridization of this atom will be sp3d2. So, its shape should be square pyramidal. Its structure can be drawn as below.
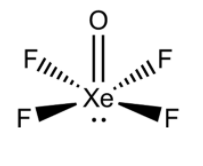
ii) H3PO3:
The name of this acid is phosphorus acid. This acid is a diprotic acid. This means that this acid gives two protons upon its full dissociation into ions in water.
- We know that the electronic configuration of P is [Ne]3s23p3.
- From the electronic configuration, we can say that it can make three sigma bonds. It also has a lone pair.
- So, in its structure, it forms a double bond with an oxygen atom in order to complete its octet. It forms a sigma bond with two –OH groups and one H atom. Thus, this structure can explain its dibasic nature. The structure can be drawn as
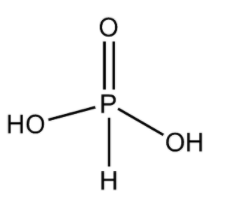
Note:
Remember that as the formula of phosphorus acid is H3PO3, it does not have three –OH groups singly bonded to one P atom. Actually, this structure is not stable and thus H3PO3 exists in the structure given just above.
