Question
Question: Draw the structure of the following compounds. (A) Ethanoic Acid (B) Bromopentane (C) Hexanol...
Draw the structure of the following compounds.
(A) Ethanoic Acid
(B) Bromopentane
(C) Hexanol
Solution
In order to draw the structure we need to identify the functional groups present in the given compounds and then draw accordingly by selecting the longest carbon chain present and position of substituted groups on the compounds.
Complete answer:
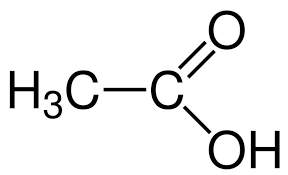
(A) Ethanoic Acid also known as acetic acid is the second member of the homologous series of carboxylic acid with −COOHas the functional group. Its molecular formula is CH3COOH. It is a weak acid that means it does not dissociate completely.
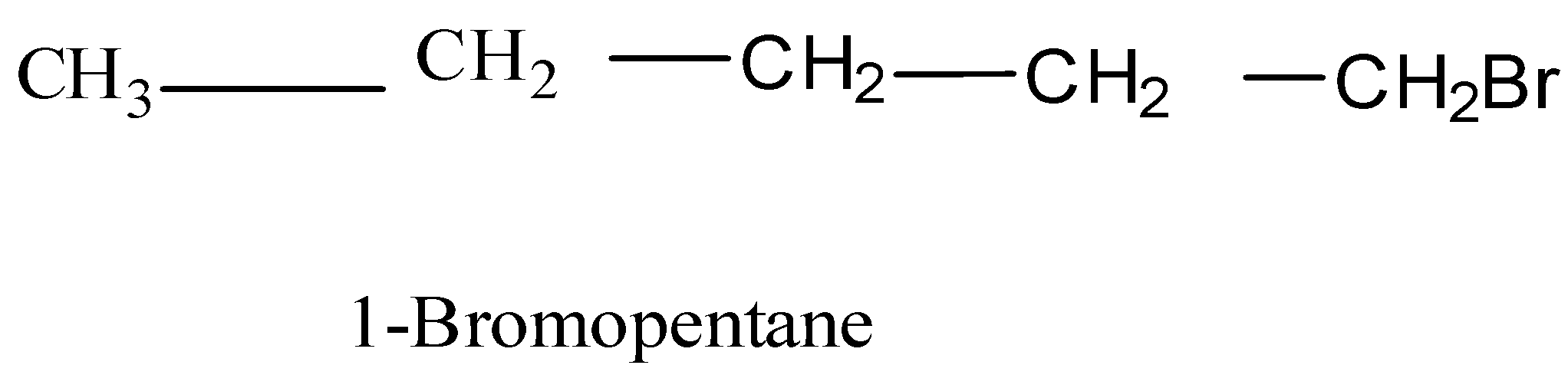
(B) Bromopentane:
To obtain the structural formula, the comprising elements and their number is needed. This can be obtained from the formula given. In the given case bromopentane which means: Pentane - The longest chain is of five carbon atoms with single bonds between them and Bromo - substituent group Bromine is present at the first carbon. Its structure formula will be:
(c) Hexanol:
To obtain the structural formula, the comprising elements and their number is needed. This can be obtained from the formula given. In the given case Hexanol which means: Hexane - The longest chain is of six carbon atoms with single bonds between them and ol(aldehyde)- substituent group −CHO is present at the first carbon. Its structure formula will be:
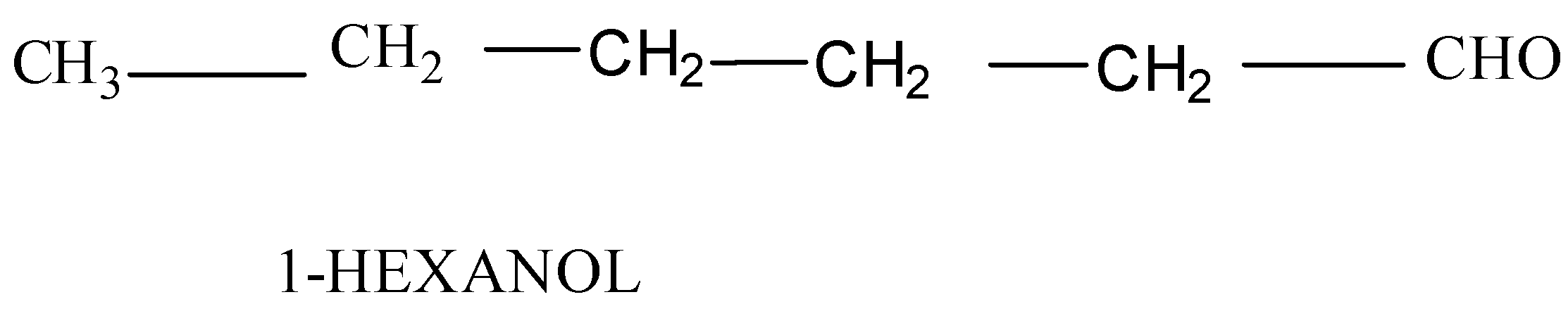
Note: While writing the structure of any organic compound two things must be taken care of. One is a substituted group on the given compound and the other is the main/functional group. The positions and alphabetically arrangement is a must to check, in order to draw the correct structural formula.
