Question
Question: Draw the structure of the following compound: 2, 2 – Dimethylpentane...
Draw the structure of the following compound: 2, 2 – Dimethylpentane
Solution
The given compound is one of the isomers of heptane which is known as neo-heptane. It contains one quaternary carbon group at one end of the carbon chain. The molecular formula is C7H16.
Complete step by step answer: 2, 2 – Dimethylpentane is an unsaturated hydrocarbon that is made up of a long-chain of 7 carbon atoms. But the parent carbon chain or the longest carbon chain consists of only 5 carbons. The other 2 carbons are present as the methyl groups.
According to the nomenclature of the compound, the parent hydrocarbon chain is pentane and the two methyl groups are attached to the second carbon of the parent hydrocarbon chain.
To draw the structure of the given compound, start by numbering the parent carbon chain.
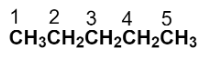
Now, we have to replace the two hydrogens with two methyl groups at the carbon numbered as 2.
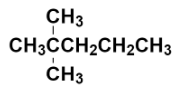
This is the structure of 2, 2 – Dimethylpentane. It is also known as neo-heptane because it is an isomer of heptane containing neo ((CH3)3C−) group.
Hence, the structure of 2, 2 – Dimethylpentane is:
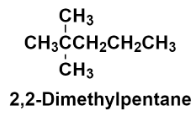
Additional information:
2, 2 – Dimethylpentane is an odorless and colorless liquid and found in a very low amount of about 0.01 in some crude oils
Note: 2, 2 – Dimethylpentane does not contain any tertiary carbon that is attached to three carbon atoms and one hydrogen and that is one of the reasons why it does not react with nitric acid.
