Question
Question: Draw the structure of the following a. Orthophosphoric acid b. Resonance structure of nitric a...
Draw the structure of the following
a. Orthophosphoric acid
b. Resonance structure of nitric acid
Solution
. To draw the structures of the given compounds, we must have a basic idea of the requirements to draw a proper structure. The basic requirements are number of covalent bonds, coordinate bonds, lone pairs of electrons, etc.
Also, to draw resonating structures, presence of lone pairs and the possibilities must be taken into account.
Complete step by step answer:
Let us now draw the structures by considering all the points required to draw the same.
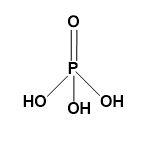
Orthophosphoric acid-
Orthophosphoric acid is the phosphoric acid with chemical formula H3PO4. It is a weak acid containing three atoms of hydrogen, one atom of phosphorus and four atoms of oxygen.
Preparation of orthophosphoric acid-
-Red phosphorus when heated with concentrated HNO3 gives orthophosphoric acid.
P+5HNO3→H3PO4+H2O+5NO2
-On large scale, the same is prepared by treating phosphorite rock with dilute H2SO4.
Ca3(PO4)2+3H2SO4→3CaSO4+2H3PO4
In short, orthophosphoric acid is the oxoacid of phosphorus. It is a tribasic acid with a double bond between P and O.
Structure-
Resonance structure of nitric acid-
Nitric acid is a strong acid with the chemical formula as HNO3. It has one hydrogen atom, a nitrogen atom and three oxygen atoms.
Preparation-
-When potassium nitrate is mixed with concentrated sulphuric acid at 200∘C, displacement reaction takes place and nitric acid is formed as,
KNO3+H2SO4→KHSO4+HNO3
-It is manufactured on a large scale by catalytic oxidation of ammonia.
4NH3+O2→4NO+6H2O2NO+O2→2NO23NO2+H2O→2HNO3+NO
In short, it is a planar molecule having two resonating structures as shown,
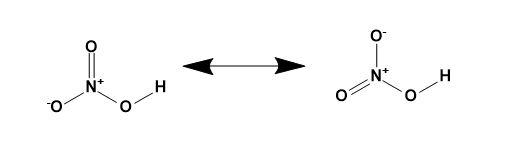
Note: The structures drawn are based on the geometry and the types of bond present in the molecule. The resonance in the nitric acid is present due the presence of oxygen atom with its lone pair of electrons.
