Question
Question: Draw the structure of\[I{{F}_{7}}\] . Write its geometry and the type of hybridization....
Draw the structure ofIF7 . Write its geometry and the type of hybridization.
Solution
It is a type of interhalogen. Its shape is obtained based on the VSEPR theory. Its geometry has two types of bond angles between elements. For finding the shape of the compound the number of total number of electron pairs and number of lone pairs should be calculated.
Complete step by step answer:
According to the VSEPR theory, the shape of the molecule is predicted by the total number of electron pairs (lone pairs + bond pairs) in the valence shell of the central I(iodine) atom.
To calculate the total number of electron pairs:
2valence electrons of central atom + number of bonded atoms
With the above formula: 27+7=7
hence, there are 7 electron pairs.
Since there are 7 fluorine atoms joined to iodine. So, there will be a 7bond pair of electrons.
Now for calculating the number of lone pairs in the compound: -
total number of electron pairs –number of bond pairs.
Lone pairs= 7−7=0.
Hence, in the compound, there are 0 lone pairs.
Now, for hybridization:
Depending on the number of I−Fcovalent bonds to be formed, the requisite number of electrons of the 5p−orbitalvalence shell of I get unpaired and promoted to the vacant 5d−orbitalsfollowed by hybridization.
All interhalogen compounds of the type XX7′involvesp3d3 hybridization of the central halogen atom.
So, the hybridization issp3d3 and it has 0 lone pairs, the geometry ofIF7 is Pentagonal bipyramidal.
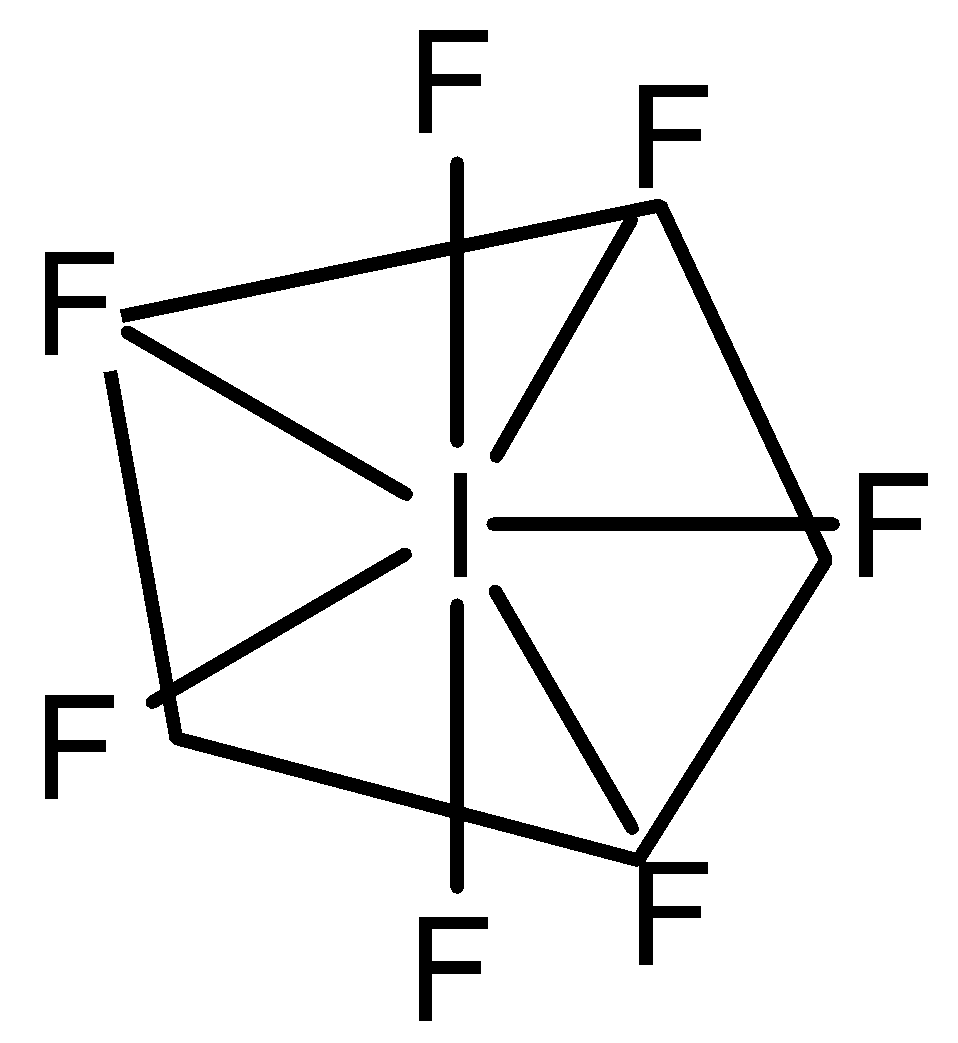
Note: Both the elements belong to the same group i.e., halogen group. The number of lone pairs should also be taken into consideration because the presence of lone pairs changes the shape and geometry completely. It has 2 bond angles, i.e., 90∘ and 72∘
