Question
Question: Draw the structure of \({{H}_{2}}S{{O}_{4}},Cr{{O}_{4}}\text{ }\) and NO and also mention the oxidat...
Draw the structure of H2SO4,CrO4 and NO and also mention the oxidation state of each atom.
Solution
To answer this question, we should know about steps to draw the structure. We should first calculate the valence electrons of each atom and then we can draw the structure.
Complete Solution :
-When we read the question, it asked us to draw the structure of H2SO4,CrO4 and NO. After that, it asked us to calculate the oxidation state of each atom. So, at first, we will draw the structure.
-To draw the structure, we will follow some steps. We will use these steps in making the structure of H2SO4,CrO4 and NO.
(i) We will first take H2SO4and draw the structure of it. For it, we should first calculate the total number of valence electrons from all the atoms in the molecule of H2SO4.
So, the total number of valence electrons in H2SO4=(1×2)+(6)+(4×6)=32 valence electrons
(ii) Then after that, we will draw the skeleton structure. In this, we will attach the atoms with single bonds in the most symmetric way possible.
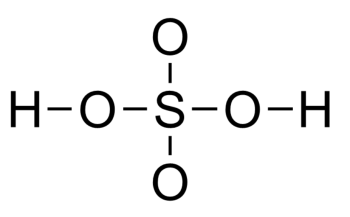
(iii) Then we will subtract the number of electrons used to make the skeleton structure from the total number of valence electrons.
So, it takes 12 electrons to draw 6 bonds. Now, after subtraction, it will be 32 – 12 = 20 electrons.
(iv) The remaining electrons as lone pairs as evenly as possible on all atoms except hydrogen. We should note that oxygen will make a double bond when it is attached singly to the central atom. Now, the final structure of H2SO4 will look like this:
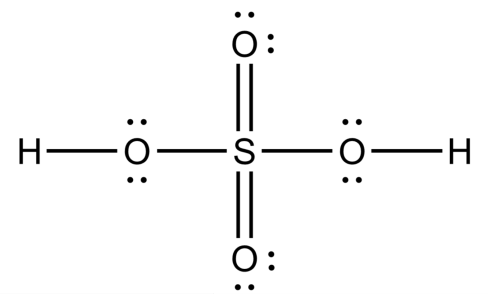
-Now, we will calculate the oxidation state of each atom in H2SO4.
Oxidation number of H = +1
Oxidation number of O = -2
Let the oxidation number of sulphur be x. Then,
2×(+1)+x+4×(−2)=0+2+x−8=0⇒x=+6
Thus the oxidation number of sulphur in H2SO4 is +6.
-Now, in the same way, we will draw the structure of CrO5 and NO.
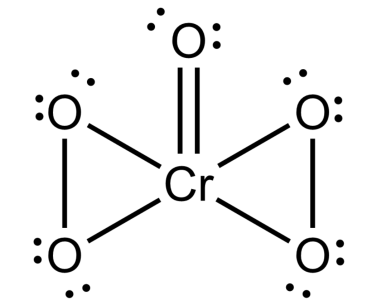
-Structure of CrO5.
-To calculate oxidation state, we should note that oxygen has an oxidation state of (-2) stated unless it's not in a peroxide. If it is peroxide then it is -1. So, we know that so if the formula is CrO5. Then 4 peroxides (-1) and one oxygen (-2)
Let us assume the oxidation state of chromium is x.
x+(−2)+4(−1)=0x−6=0⇒x=+6
Hence, the chromium oxidation number is +6
-Now, we will draw the structure of NO.
We should note that in the structure of nitric oxide, triple electrons will be placed on nitrogen.
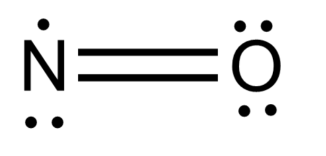
-For calculating the oxidation state of nitrogen, we should note that O always has an oxidation state of -2, and since NO is neutral, N must have an oxidation state as follows:
Let us assume the oxidation state of Nitrogen is x.
x+(−2)=0⇒x=+2
So, the oxidation state of nitrogen is +2.
So in the above part, we had successfully drawn the structure of H2SO4,CrO4 and NO and calculated the oxidation state.
Note: We should know the difference between charge and oxidation number. We should note that a charge is when an element gains or loses an electron, causing it to be positive or negative. We don’t need to worry too much about the oxidation numbers of ionic compounds because they are the same as the ion’s charge. For atoms that are bonded covalently, oxidation numbers are a sort of imaginary charge, as if the molecule was bonded ionically.
