Question
Question: Draw the structure of following compound \[{{N}}{{{a}}_{{6}}}{{{P}}_{{6}}}{{{O}}_{{{18}}}}\]....
Draw the structure of following compound Na6P6O18.
Solution
Na6P6O18 is sodium metaphosphate. It is a type of coordinate compound. Coordinate compounds usually contain coordinate bonds in them. It is a mixture of polymeric metaphosphates and is made by the composition of monomers.
Complete step by step answer:
Na6P6O18, commonly called as sodium metaphosphate is a hexamer of the monomer (NaPO3)6. Its correct technical name is sodium poly-metaphosphate. It is also known as Graham’s salt, SHMP etc. As we said that it is a hexamer, it’s structure can be shown as:
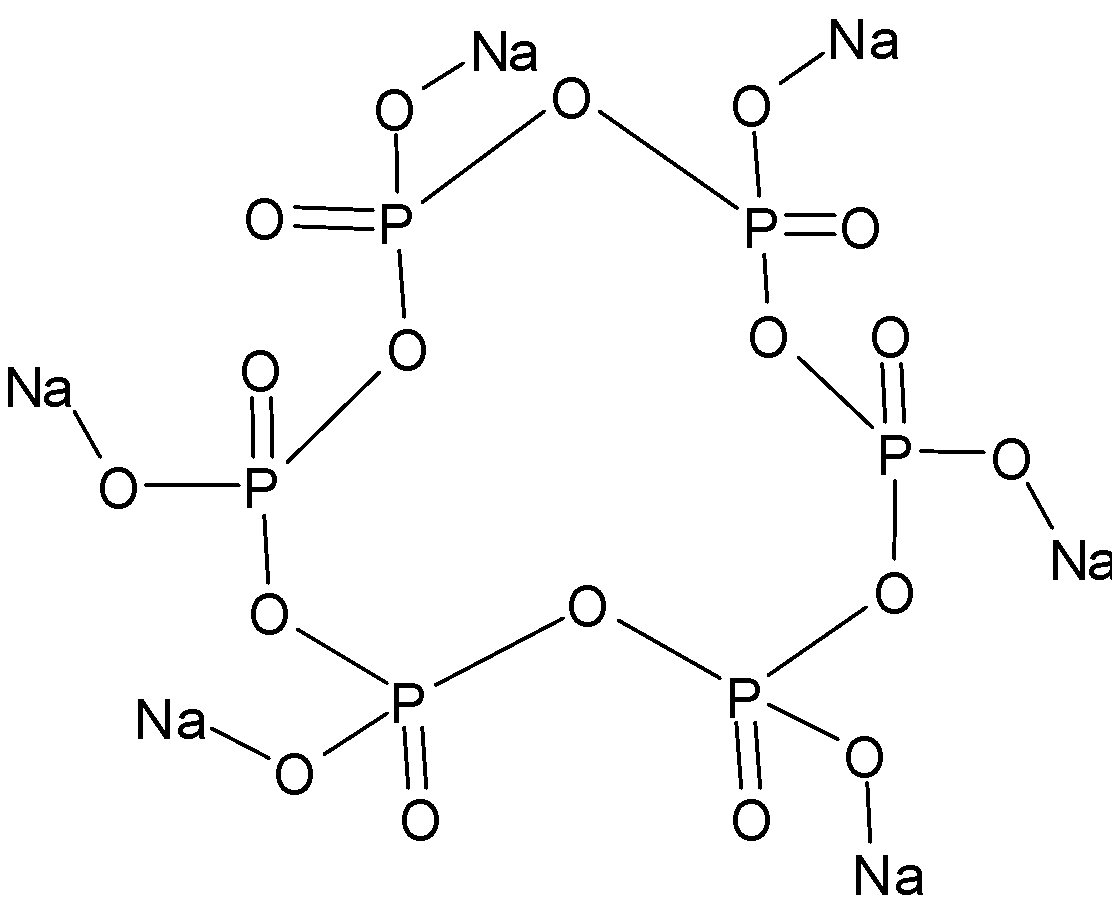
The significant uses of the compound include its use as a deflocculant in the production of clay-based ceramic particles. It can also be used as a dispersing agent to break down clay and other soil types for the assessment of soil texture for agricultural purposes. Its other uses include its addition in toothpastes to prevent staining in which it acts as an anti-staining agent and is also added for the prevention of tartar formation.
Its main use is as a food additive. It increases the quality and stability of food items. It can also be considered as a preservative. As it is a chelate compound, it prevents the oxidation of fat present in the food items as it contains polyvalent metal ions. When added to a food item, it acts as an emulsifier. It is used in fish fillets, fruit jellies, ice creams etc.
Additional information:
The compound has a mild corrosive nature and can be irritating to skin. Also high concentrations of the compound may cause acute side effects such as irregular pulse and so on because of the high concentration of sodium.
Note: Sodium metaphosphate is a hexamer of (NaPO3)6. It is a chelating ligand, that is, it has more than one point of attachment to the metal ion in its structure. They are also known as polydentate ligands because of the presence of more than one point of attachment.
