Question
Question: Draw the structure of boric acid. From the structure of boric acid, show that it is monobasic acid....
Draw the structure of boric acid. From the structure of boric acid, show that it is monobasic acid.
Solution
Here we will proceed by drawing the structure of boric acid using atoms of phosphorus, and three atoms of hydrogen as its components. Then using the structure and composition of boric acid, we will prove that it is monobasic acid.
Complete step by step answer:
Boric acid is a monobasic Lewis acid with the chemical formula: H3BO3. It is an acid-containing four atoms of phosphorus, and three atoms of hydrogen. Boric acid is also known as hydrogen borate, boracic acid, and ortho-boric acid.
Boric acid can be prepared by reacting borax with hydrochloric acid. It should be noted that Wilhelm Homberg was the first person to prepare boric acid from borax. Boric acid is soluble in water and does not have any characteristic odour. The molecular weight is 61.83 grams. The boiling point of boric acid is 158 degrees Celsius. The melting point of boric acid is 300 degrees Celsius. The conjugate base of boric acid is the borate anion.
Preparation of Boric acid-
One of the simplest methods of preparing boric acid is by reacting borax with any mineral acid (hydrochloric acid). Boric acid can also be prepared from the hydrolysis of diborane and trihalides of boron (such as boron trichloride or boron trifluoride).
Structure of H3BO3 molecules is as shown as in the below figure:
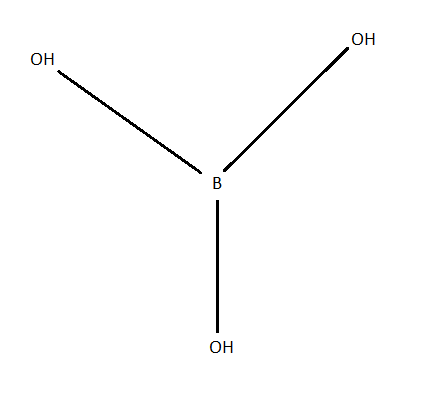
Each boric acid molecule features boron-oxygen single bonds. The boron atom occupies the central position and is linked to three hydroxyl groups. The overall molecular geometry of boric acid is trigonal planar.
Using the structure of H3BO3, we can see that boric acid contains −OH groups yet it acts as monobasic acid rather than tribasic acid because boric acid does not act as proton donor but it accepts a lone pair of electrons from OH negative ions and forms B(OH)4−. Also, boric acid behaves as Lewis acid because it accepts a lone pair of electrons from OH negative ions from water.
Note: Whenever we face such type of question, one should know all the components of boric acid (boron-oxygen single bonds) to form structure of boric acid (H3BO3). Also, one should not get confused whether the boric acid is monobasic acid rather than tribasic acid.
