Question
Question: Draw the structure of \(BeC{l_2}\) (Vapour)....
Draw the structure of BeCl2 (Vapour).
Solution
There are three lone pairs in the chlorine atom even after it forms a covalent bond with Beryllium. The lone pairs need to be considered in defining the structure.
Complete step by step answer:
There are two states in which the BeCl2molecules exist in nature. One is the vapour phase and another one is the solid phase. The vapour phase is the phase in which the molecules are present in the form of a dimer. This means two molecules of BeCl2 are there which form internal bonds between the structure and ultimately result in the formation of a molecule when in the form of a vapour. The chemical structure of the molecule when it exists in vapour form is known as a dimer form because two monomeric structures are linked together. The chemical structure of the molecule is depicted below:
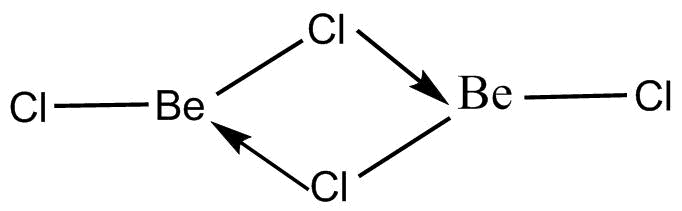
The chemical structure shows the two monomers of BeCl2 together arranged in the manner of a dimer. There are three lone pairs in Chlorine even after the formation of bonds with Beryllium. Thus, one of the lone pairs can be donated to the vacant orbitals of Beryllium. This donation of lone pair electrons results in the formation of coordinate bonds.
Therefore, two coordinate bonds are formed resulting in the structure of BeCl2in vapour state. Therefore, the actual molecule exists as a Be2Cl4 state which forms a single molecule in the state of a dimer. This structure is present in the vapour phase so that the stability can be attained in the major structure.
Note: While defining the structure the electronic configuration of both the chlorine atom and beryllium atom needs to be understood. Formation of this structure depends on the presence of excess electrons in each of the atoms.
