Question
Question: Draw the structure of: A.Pyrophosphoric acid B.Pyro phosphorous acid C.Poly metaphosphoric aci...
Draw the structure of:
A.Pyrophosphoric acid
B.Pyro phosphorous acid
C.Poly metaphosphoric acid
D.Cyclo trimetaphosphoric acid
Solution
To answer this question, you should recall the concept of oxoacids of phosphorus. Phosphorus belongs to group 15 of the periodic table and forms various oxoacids: hypo phosphorous acid(H3PO2), Phosphorous acid(H3PO3), Hypo phosphoric acid(H3PO4), pyro phosphoric acid (H4P2O7)and poly metaphosphoric acid(HPO3)n.
Complete Step by step solution:
Oxoacids of phosphorus which contain phosphorus-oxygen linkages are the most dominated subset in Phosphorus Chemistry.
Pyro phosphoric acid is a tetrabasic acid and prepared by heating orthophosphoric acid at about 250oC : 2H3PO4→H4P2O7+H2O The structure of Pyro phosphoric acid can be represented as:
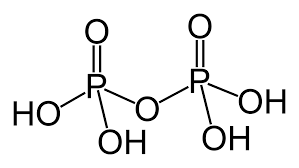
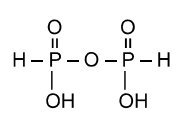
The chemical formula of pyrophosphoric acid is H4P2O5. The structure can be represented as:
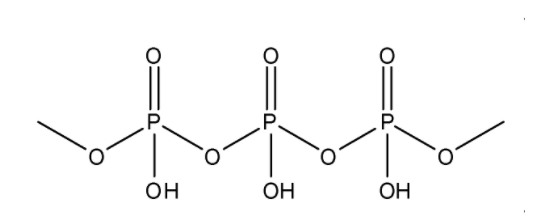
The formula for Poly metaphosphoric acid is (HPO3)n. It has a linear polymer structure where the oxidation state of phosphorus is +5. The structure can be represented as:
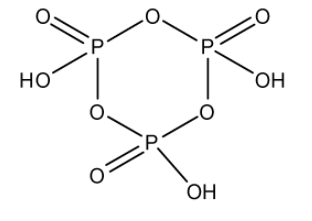
Cyclo trimetaphosphoric acid is a tribasic acid of phosphorus which has a formula (HPO3)3. The three metaphosphoric acid molecules form a ring structure. Phosphorus is in the +5 oxidation state. In this structure, there are three P−OH bonds, three P=O bonds, and three P−O−P bonds:
Note: Important acidic nature of oxoacids of phosphorus: You are already aware of the term acids. These acids show varied physical and chemical properties of acids. Some simple ones are – they need a pH below 7, they turn blue paper red, they need a sour taste and that they react with alkalis to make salts. There is little difference of acidic strength among H3PO4, H3PO3 and H3PO2 because the hydrogen in these acids aren't all bonded to oxygen, and phosphorus isn't a highly electronegative element i.e. its oxides are less basic and electronegativities of P and H are almost the same. Hence, they all have almost similar acidic strength.

