Question
Question: Draw the structure of A. 
Solution
We know that the carbonyl carbon is formed by the interaction of carbon with oxygen via double bond. Carbon is less electronegative than oxygen, due to which electron density gets shifted towards oxygen. There is a formation of polarity in the overall molecule.carbonyl carbon shows resonance with oxygen imparting extra stability and more acidic character.
Complete solution:
The pKa value is about 50−60 for normal alkyl C−H bonds, while the pKa value is about19−20 for alpha hydrogen to the carbonyl group. The hydrogen atom which is at alpha position to the carbonyl group is acidic in nature. This can be also explained on the stabilization of carbanion by the resonance. So, this hydrogen is prone to any base.
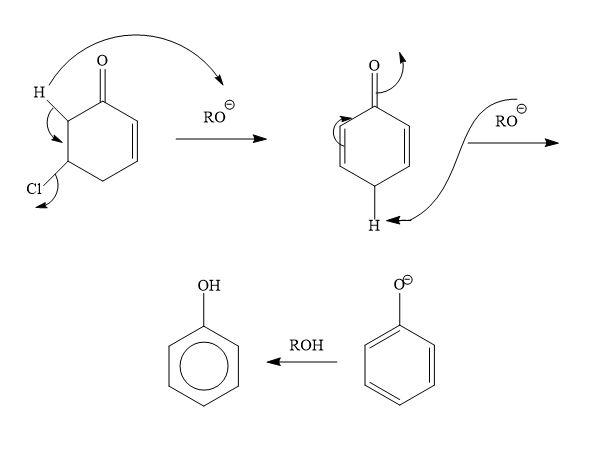
In our reaction, tertiary butoxide acts as a base. So, any hydrogen which is acidic is taken up by this group. In our reactant, there is presence of alpha hydrogen which is acidic in nature so it is taken off by the base. And negative charge will develop on the alpha carbon. In the next step, this negative charge will have migrated to the carbon containing chloride and it will form a double bond with chlorine bringing better leaving groups as chloride ions. This chloride ion combines with acidic hydrogen to form hydrochloric acid. Then the molecule can be easily aromatised by taking another acidic hydrogen by the base present in the reaction. Due to which there is a formation of phenoxide ions. Then after addition of proton phenol is formed as the final product.
Note: Since in the carbonyl group there is an overlapping of carbonyl pi bond with the unhybridized p atomic orbitals. Due to which the alpha hydrogens are more acidic in nature as pi orbitals are involved in conjugation.this conjugation also describes the extra stability to the carbonyl carbon.
