Question
Question: Draw the structural formula of Ethanoic acid....
Draw the structural formula of Ethanoic acid.
Solution
Hint: Solve this question by breaking up the name of the given organic compound. The key words used in the compound will tell you about the number of carbons, if the compound is an alkane, alkene or alkyne and its functional group.
Complete step by step answer:
Ethanoic acid is a colourless clear liquid with a pungent and a characteristic odour. Being an acid, it is a proton donor.
As the name suggests, ethanoic acid is composed of three key words, i.e. eth + ane + oic acid.
‘eth’ indicates that the compound contains two carbons
The compound becomes –
C-C
‘ane’ indicates that the compound is an alkane, i.e. it is a saturated compound containing no double or triple bonds.
The compound becomes –
H3C−CH3
‘oic acid’ indicates that this compound contains a carboxylic acid group (-COOH)
The compound becomes –
H3C−COOH
Therefore, the structural formula of Ethanoic acid H3C−COOH is -
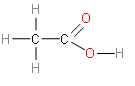
Additional Information:
Ethanoic acid can be prepared by oxidation of ethanol. Oxidation is a method by which an oxygen is added to a compound or hydrogen is removed from a compound, or both.
Note: Let us understand the following terms –
“Alkanes are the simplest family of hydrocarbons - compounds containing carbon and hydrogen only. They only contain carbon-hydrogen bonds and carbon-carbon single bonds.”
“Carboxylic acids are compounds which contain a -COOH group.”
“Alcohols are compounds in which one or more hydrogen atoms in an alkane have been replaced by an -OH group.”
