Question
Question: Draw the structural formula of ethane. Draw electron-dot structure of propane....
Draw the structural formula of ethane. Draw electron-dot structure of propane.
Solution
Carbon has a valency of four, so four groups or atoms can be attached to one carbon atom. Also, a single bond is formed by the contribution of two electrons which are mutually shared between two atoms.
Complete answer:
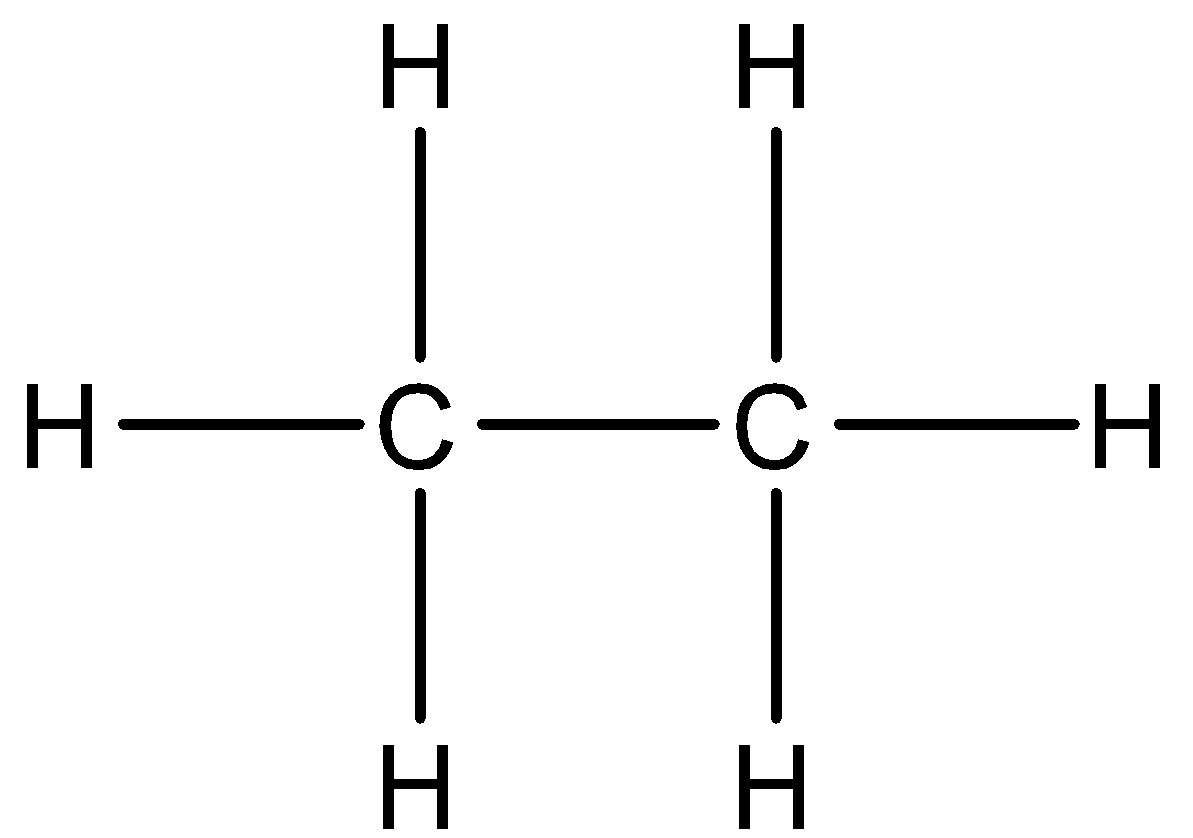
In order to answer the question, let us know about the bonding properties of carbon. Carbon is a tetravalent atom. It means that a single carbon atom can bond with only four other carbon atoms or other atoms or groups. It also has the property of catenation, which means that carbon can form very long chains. Now, the valency of the carbon atom can be satisfied by atoms, groups as well as electrons. A bond is formed when two atoms have a mutual sharing of electrons. Now, let us answer the question. Ethane has the chemical formula of C2H6, which means that it has two carbon atoms and six hydrogen atoms. The suffix “ane” indicates that compound has no unsaturation, so double bond is not present, and all the four valency of carbon are satisfied. So, the correct structure of ethane would be:
In the second part of the question, we have been asked about the electron dot structure of propane. In electron dot structure, each electron is represented by a dot. As a single bond consists of two electrons, which are mutually shared between the atoms, so in order to represent a single bond, we will use two electron dots in the diagram. Similarly, for double bonds, we will draw four electron dots. Propanal has the chemical formula of C3H8 and it contains no unsaturation. So the electron dot structure would be:
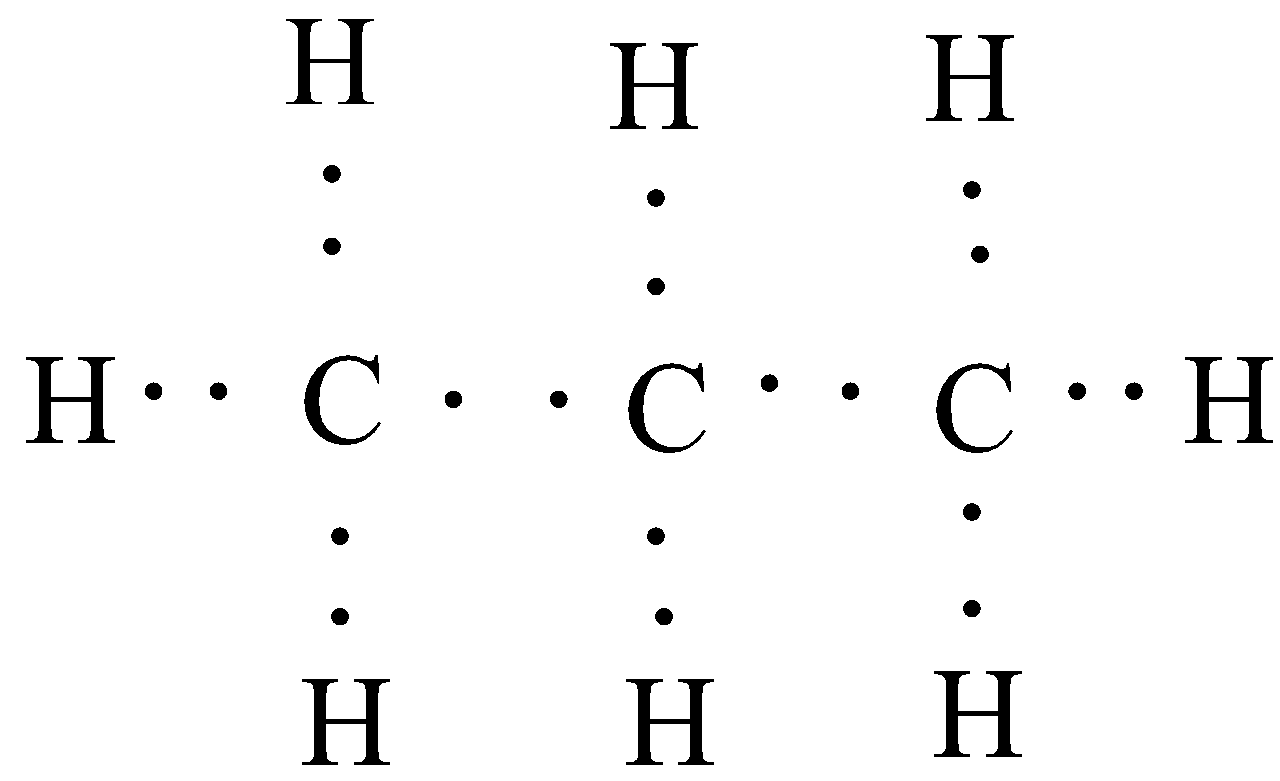
Hence, we obtain the required structures for ethane and propane.
Note:
It should be noted that in the structures which contain resonance, the hybrid structure should be drawn, which is a combination of all the possible resonating forms.
