Question
Question: Draw the shape of five d-orbitals....
Draw the shape of five d-orbitals.
Solution
. Atomic orbitals:- The three-dimensional space around the atom of an element in which the electron density is maximum. It means the probability of finding an electron is maximum.
Complete step by step answer:
The transition metal ions of the outermost d-orbitals are not completely filled with electrons and hence, they can easily take and give electrons.
For d-orbitals the value of l = 2 , so, the value of m will be: −lto+l.(−2,−1,0,+1,+2).
As we see that there are five magnetic quantum numbers, so, the d-orbital will have five orientations. It means, five types of shapes of d-orbital in three-dimensional space.
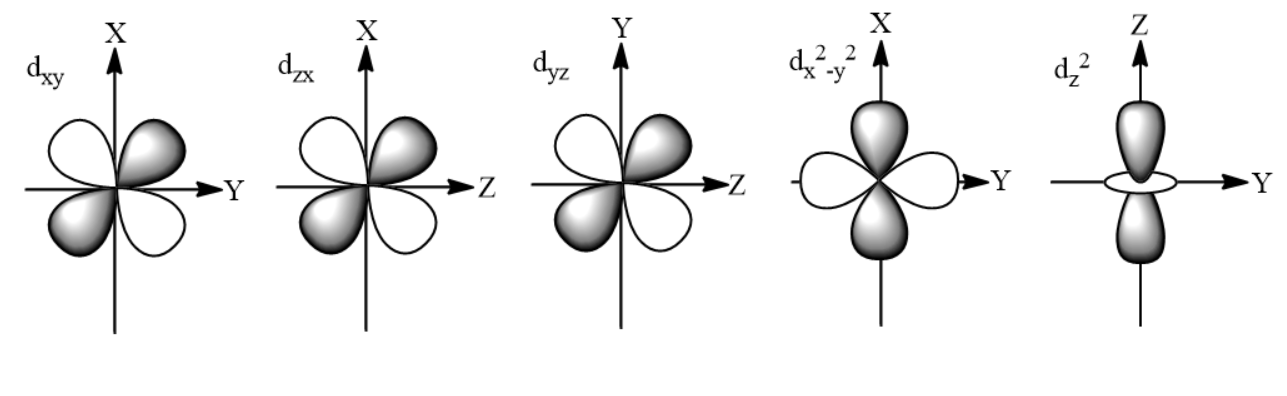
So, depending upon the axes along which or between which the electron clouds are obtained, different names and shapes are given as;
dxy = It is of cloverleaf-like shape.
dyz = It is of cloverleaf-like shape.
dxz = It is of cloverleaf-like shape.
dx2−y2 =It is of cloverleaf-like shape.
dz2 = It is of ‘dumbbell’ and ‘doughnut’ like shape. The lobes of the ‘dumbbell' lie along the z-axis and ‘doughnut’ lies in the xy plane.
It is clear from the above names of the five d-orbitals that in which position will be assigned to them in the xyz coordinates, so the shapes are:
Additional Information:
Significance of four quantum numbers:
The principal quantum number n = It describes the energy and distance from the nucleus and represents the shell.
The azimuthal quantum number l = It describes the shape of the subshell and its orbitals.
The magnetic quantum number m = It describes the orientation of the orbitals in space.
The spin quantum number s = It describes the spin of each electron in the orbital.
Note: The possibility to make a mistake is that dx2−y2 has a cloverleaf-like shape and not dumbbell as the lobes are present in the xy plane only.
