Question
Question: Draw the resonating structures of \(\text{S}{{\text{O}}_{4}}\text{ and C}{{\text{O}}_{2}}.\)...
Draw the resonating structures of SO4 and CO2.
Solution
We know that the resonance structures are the various different structures of a Lewis structure, which describes how the negative structure is delocalized over the molecule to make the structure stable. Resonance structures are capable of describing delocalized electrons that cannot be expressed by a single Lewis formula with an integer number of covalent bonds.
Complete answer:
We know that resonance is the way by which we describe delocalized electrons within certain molecules or polyatomic ions where the bonding can’t be expressed by a single Lewis formula or structure. Resonance structures: These are the set of two or more Lewis Structures that collectively describe the electronic bonding of a single polyatomic species including partial bonds and partial charges. partial bonds and partial charges.
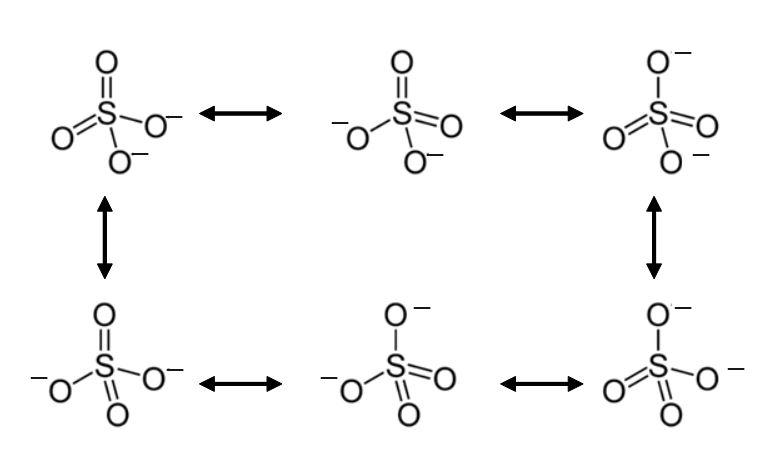
To make the resonance structure of SO4 molecule, first draw its Lewis structure. The SO4 molecule consists of two oxygens bonded by double bonds and two oxygens bonded by single bonds. The two oxygen atoms bonded by a single bond have one paired electron each. Now the oxygen’s with unpaired electrons will delocalize their negative charge to form a double bond. But the S atom can have only 2 double bonds, so to make the molecule stable the oxygen with a double bond will break its double bond to form a single bond and have a negative charge on itself. Similarly, the other oxygen double bond will try to form a double bond by delocalizing its negative charge. This way we will get 6 resonance structures of the SO4 molecule.
To make the resonance structure of CO2, the molecule first draws its Lewis structure. CO2 consists of a carbon atom bonded to two oxygen atoms by double bonds. In this molecule we do not have any unpaired electrons. But one of the oxygen tends to make a triple bond with carbon due to its formal charge, so the other oxygen atom breaks its double bond to make a single bond to acquire a negative charge and the other atom with the triple bond acquires a positive charge.
..O..=C=..O..↔+:O≡C−..O..:−
Note:
Remember that while drawing resonating structures you should be careful about lone pairs and charges. Here all double bonds partially exist. And oxygen has partial negative charge resonance structures are not actual structures, they are a concept to explain the stability of a molecular structure. Resonance is the phenomenon which lets us draw different structures.
