Question
Question: Draw the pyranose structure of glucose....
Draw the pyranose structure of glucose.
Solution
The pyranose structure of glucose is similar to the organic compound pyran, which is a six membered ring with one oxygen and five carbon atoms in the ring.
Complete answer:
In order to answer the question, we need to learn about the structure and properties of glucose.
Cyclic structure of glucose: The limitations shown by the open chain structure of glucose can be explained by its cyclic structure. It was proposed that glucose can form a six-membered ring in which −OH at C-5 can add to the −CHO group and can form a cyclic hemiacetal structure. This explains the absence of −CHO group and also the existence of glucose in α,β forms.
The two cyclic hemiacetal forms of glucose differ only in the configuration of the hydroxyl group at C-1, called anomeric carbon (the aldehyde carbon before cyclisation) and the corresponding α and β forms are called anomers. It should be noted that a and B-forms of glucose are not mirror images of each other, hence are not enantiomers. The six membered cyclic structure of glucose is called pyranose structure (α or β), in analogy with pyran.
Pyran is a six membered ring with one oxygen and five carbon atoms in the ring. The cyclic structure of glucose can be more accurately shown by Haworth structure as given below:
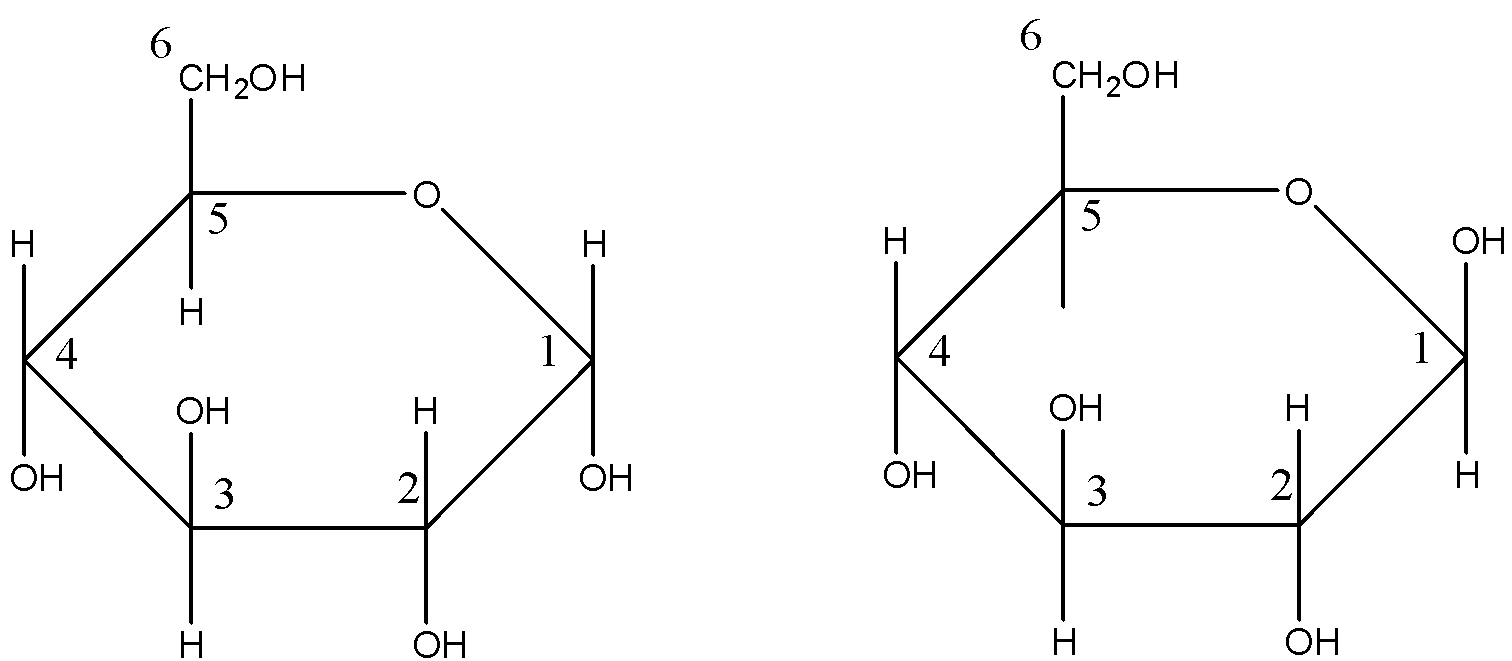
The left diagram shows the structure of α-D-( + ) Glucopyranose and the right diagram shows the structure of β-D-( + ) Glucopyranose.
Note:
It is to be noted that the cyclic structure of glucose was so given because the existence of glucose in α,β forms could not be explained by it’s open chain structure.
