Question
Question: Draw the molecular structure of the following compounds: \( {{H}_{2}}{{S}_{2}}{{O}_{8}} \)...
Draw the molecular structure of the following compounds:
H2S2O8
Solution
Hint : The H2S2O8 is known as peroxydisulfuric acid. It is known as the inorganic compound. In this compound we will have S−O−O−H linkage. In the structural form the peroxydisulfuric acid is written as the HO3SOOSO3H . It contains a peroxide group in its structure. It is one of the types of oxoacid of sulphur.
Complete Step By Step Answer:
The structure of the peroxydisulfuric acid can be drawn as the following:
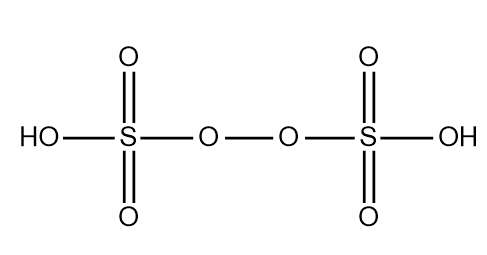
In this structure we observe a peroxide group which tends to form a bridge between the two atoms of sulphur. Both sulphur are attached to one hydroxyl group and two atoms of oxygen other than the peroxide group.
The basicity of the compound or the molecule can be determined by the number of the hydroxyl groups attached to sulphur are present in the compound which can ionise to give the hydrogen ion or the hydronium ion.
Here we have two groups of the hydroxyl attached to the sulphur so the basicity of the peroxydisulfuric acid is two. In the molecule we have the peroxide linkage through which the two atoms of the sulphur are attached. The sulphur has the oxidation state equal to +6 in the molecule.
Each atom of the sulphur in the molecule has the hybridisation equal to sp3 as the sulphur tends to form 4 bond pairs. It is a strong oxidising agent which is highly explosive in nature
So the structure of peroxydisulphuric acid is given in which the sulphur has +6 oxidation state.
Note :
The other oxoacids of sulphur are sulphuric acid, sulfurous acid, etc. the sulphuric acid has the tetrahedral geometry. It is produced by the contact process. Sulphurous acid is considered as the diprotic acid as it tends to ionize two protons. It is formed by dissolving the sulphur dioxide in water. The peroxydisulfuric acid is used in the photography as the hypo eliminator.
