Question
Question: Draw the molecular structure of\[\text{ }{{\text{N}}_{\text{2}}}{{\text{O}}_{\text{5}}}\]....
Draw the molecular structure of N2O5.
Solution
The dinitrogen pentoxide N2O5is a challenging structure. The Lewis dot structure contains the 40 valence electrons and thus a total of 20 electron-pair to accommodate the structure. This electron-pair can be sigma bonds, pi bonds, or lone pairs. Each oxygen completes its content by accommodating two pairs of two oxygen and nitrogen and completing its octet by forming a pi bond with the adjacent oxygen atom.
Complete step by step answer:
This N2O5 is a bit challenging molecule to draw.
First, we will sketch the basic structure N2O5as shown below. Now we are going to find out how the σ bonds, π bonds, and lone pairs are arranged in the molecule.
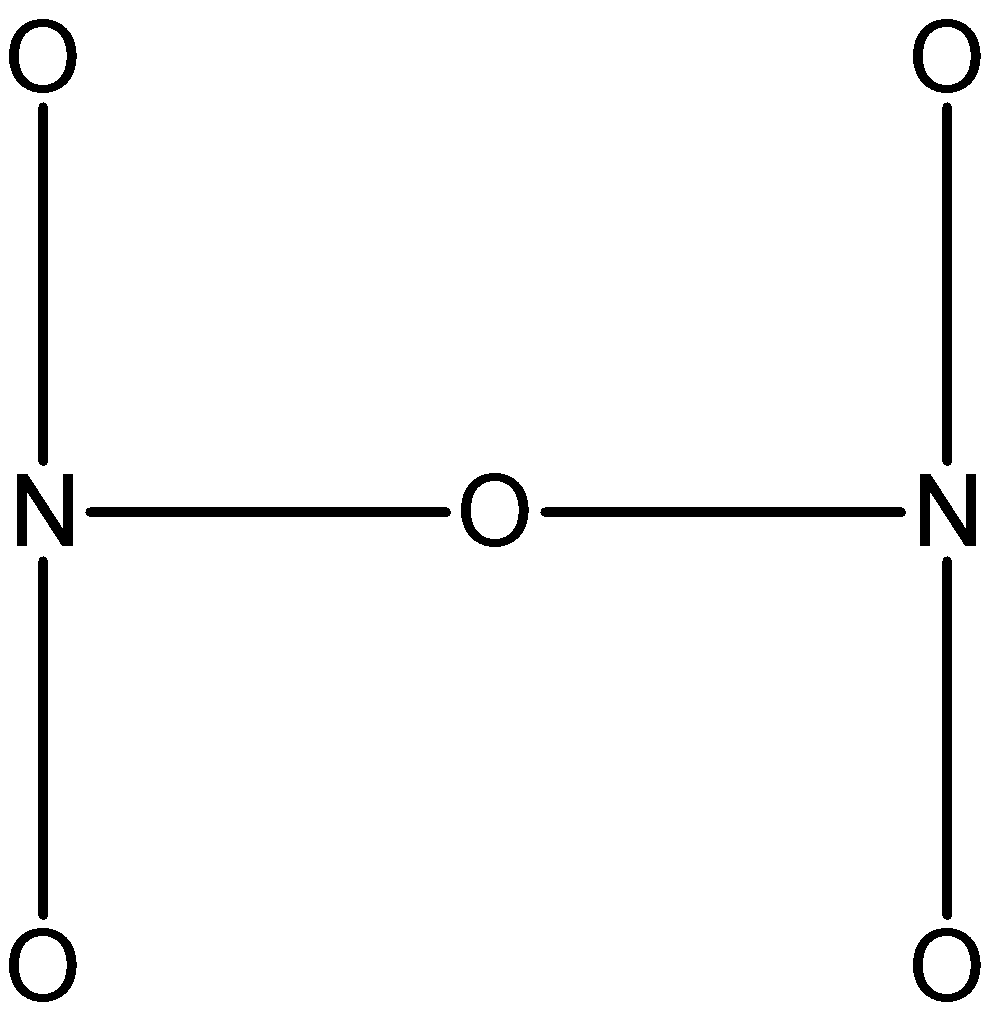
The structure is arranged such that the 5 oxygen and 2 nitrogen are forming a skeleton N2O5.
In N2O5,
The total number of the valence electron in the nitrogen atom = 5
The Number of a nitrogen atom in the N2O5molecules = 2
Therefore, the total number of valence electron from two nitrogen atoms are, 5 × 2 =10 e−
Now, each nitrogen is bonded to the oxygen.
The number of the valence electron in the oxygen atom = 6
The number of oxygen in the N2O5molecule atom = 5
Therefore, the total number of an electron from the two oxygen atoms are, 6 × 5 = 30 e−
Thus, the total number of electrons in the valence shell of each atom is,
= 10 + 30 = 40 e−
Now, we can calculate the total number of pairs of the electron. To do so, divide the total valence electron by the 2. we get,
number of electron pair = 240 e− = 20 pairs
There are 20 electron pairs in N2O5. Thus 20 electron pairs may be σ bonds, π bonds, or lone pairs.
Now, we will start to draw the Lewis dot structure for N2O5. In the above skeleton we have already marked 5 σ bonds.so we are left with 15 electron pairs. Let's mark the rest of the electron pair as the lone pairs of oxygen. The lone pairs are updated as follows,
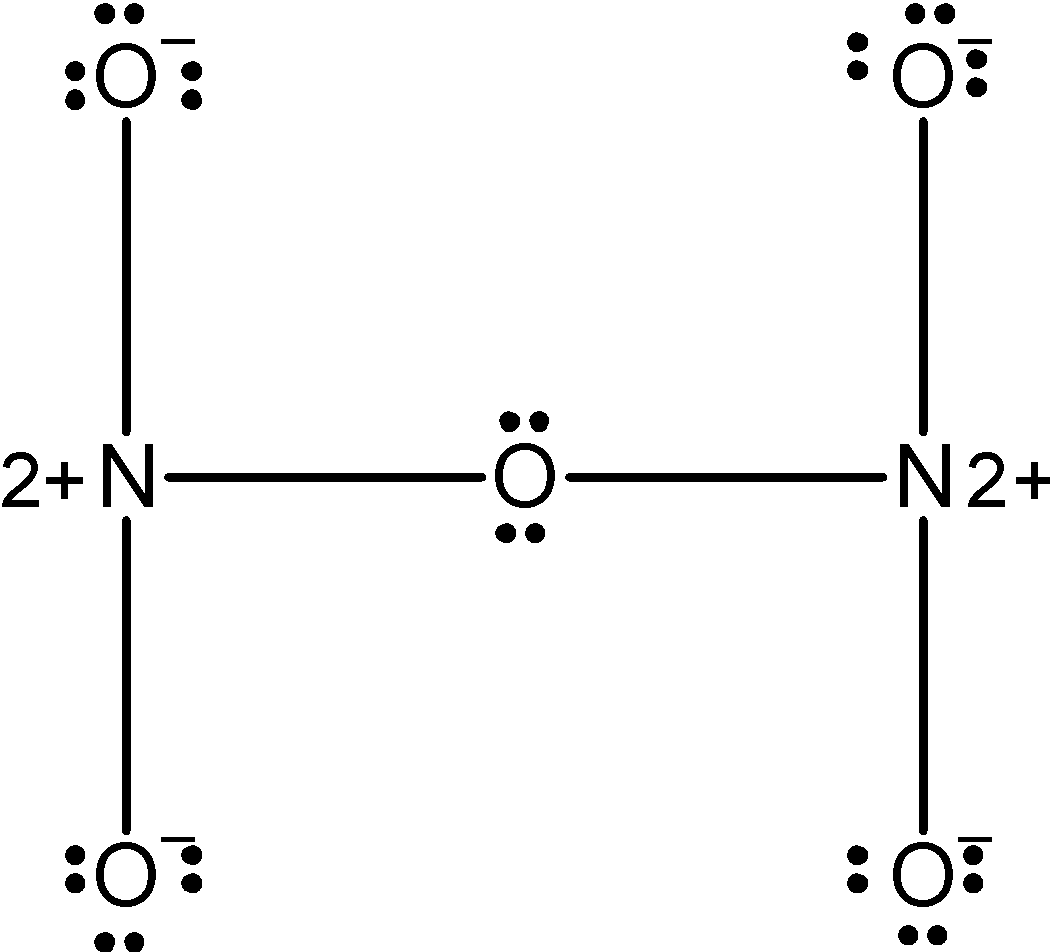
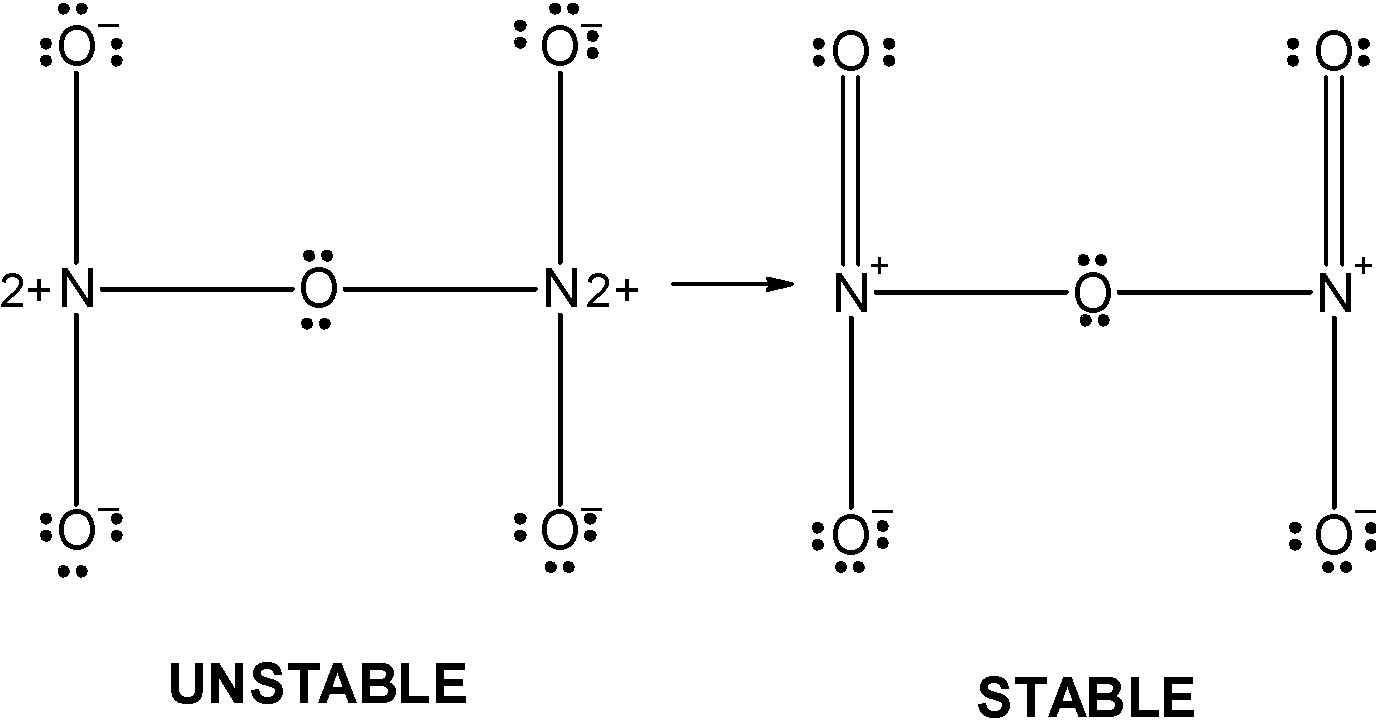
The 4 oxygen has the 8 electrons in their valence shell. They possess the -1 charge. The one oxygen which is in between the two nitrogen atoms has completed its valency and thus it has zero charges on it. The structure depicts that the octet of the nitrogen is not complete since both of them acquire the +2 charge. Therefore the above structure is wrong and unstable therefore we can reduce the charge on the atoms by converting the lone pairs on the oxygen by the pi bonds. The modified structure is as follows,
Therefore, the correct stable molecular structure of N2O5 is
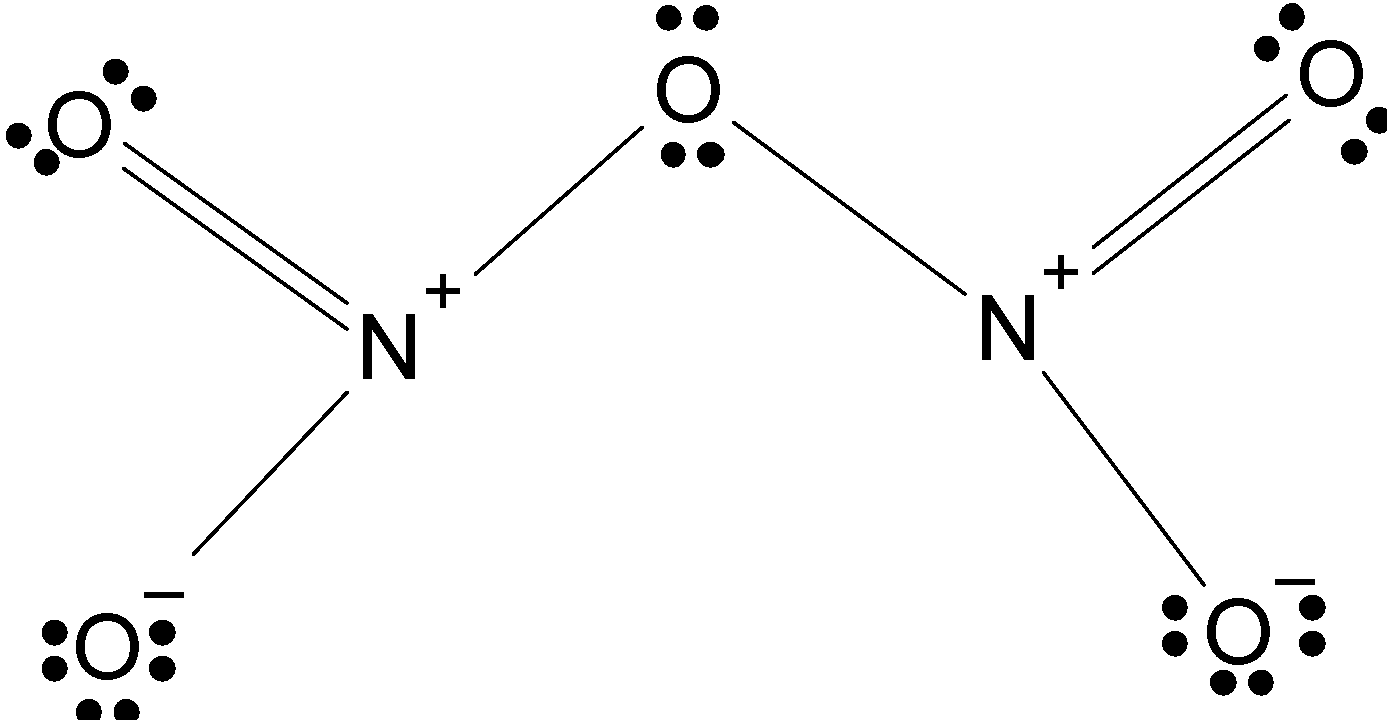
Note: The structure contains the 40 valence electron therefore it is challenging to draw the structure. The key is to place oxygen atoms between the nitrogen atoms and attach the four atoms to the nitrogen atom. This is an exception to the “least electronegative atom at the centre rule”. Thus makes its structure tricky.
