Question
Question: Draw the molecular orbital energy level diagram of \({N_2}\) molecules....
Draw the molecular orbital energy level diagram of N2 molecules.
Solution
The number of electrons present in the bonding orbitals is represented by Nb and the number of electrons present in antibonding orbitals by Na
Complete step by step answer:
Energy level diagrams are a means of analysing the energies electrons can accept and release as they transition from one accepted orbital to another
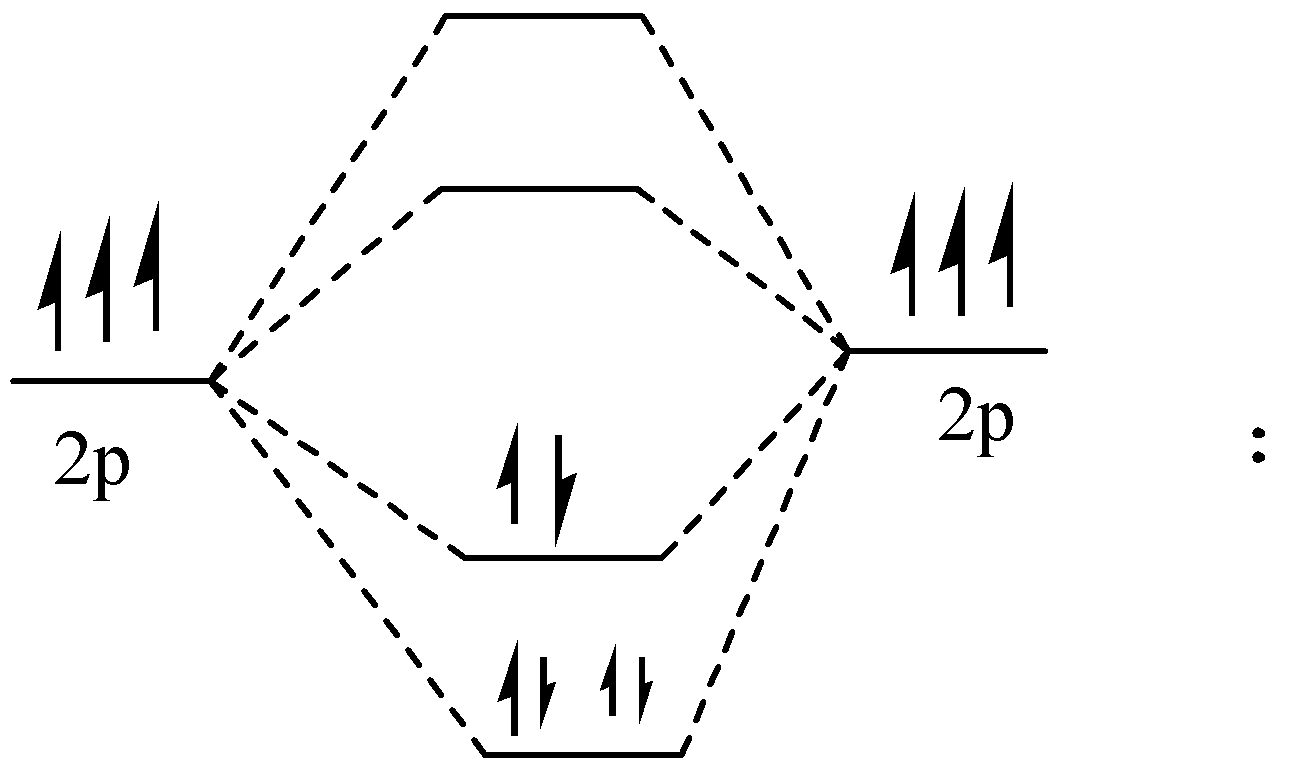
Atomic nitrogen has 5 valence electrons and 4 valence orbitals (2s, 2px , 2py , and 2pz ). In the Lewis structure there is a triple bond between the nitrogen atoms and a nonbonding pair of electrons on each. This is consistent with the physical properties of N2 .
σ∗2p
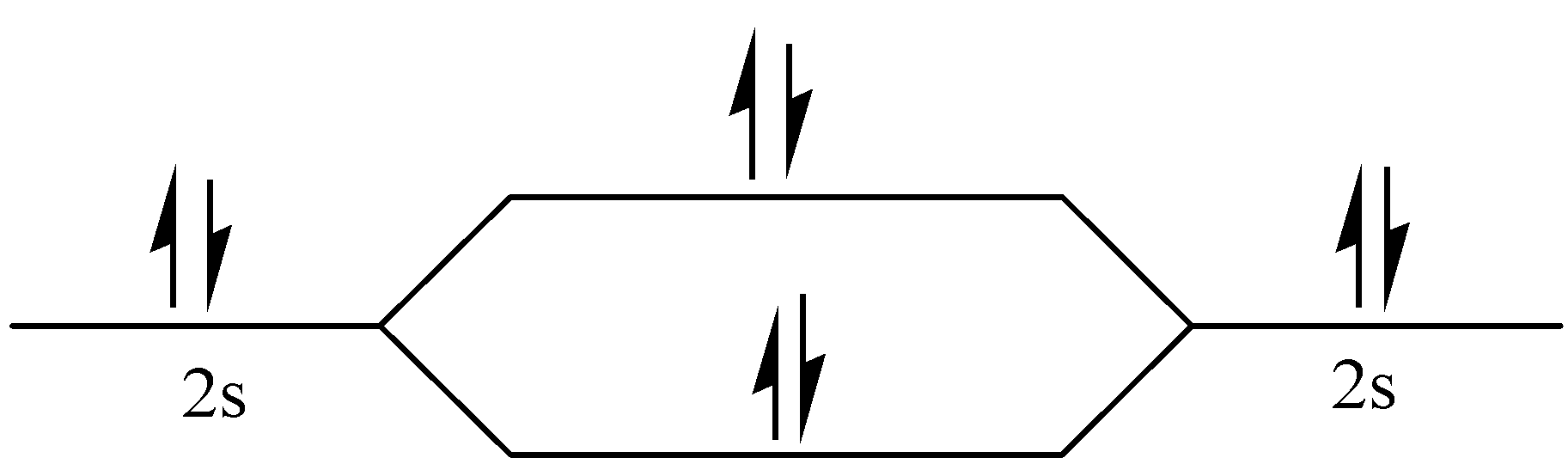
σ2s∗
σ2s
σ1s∗
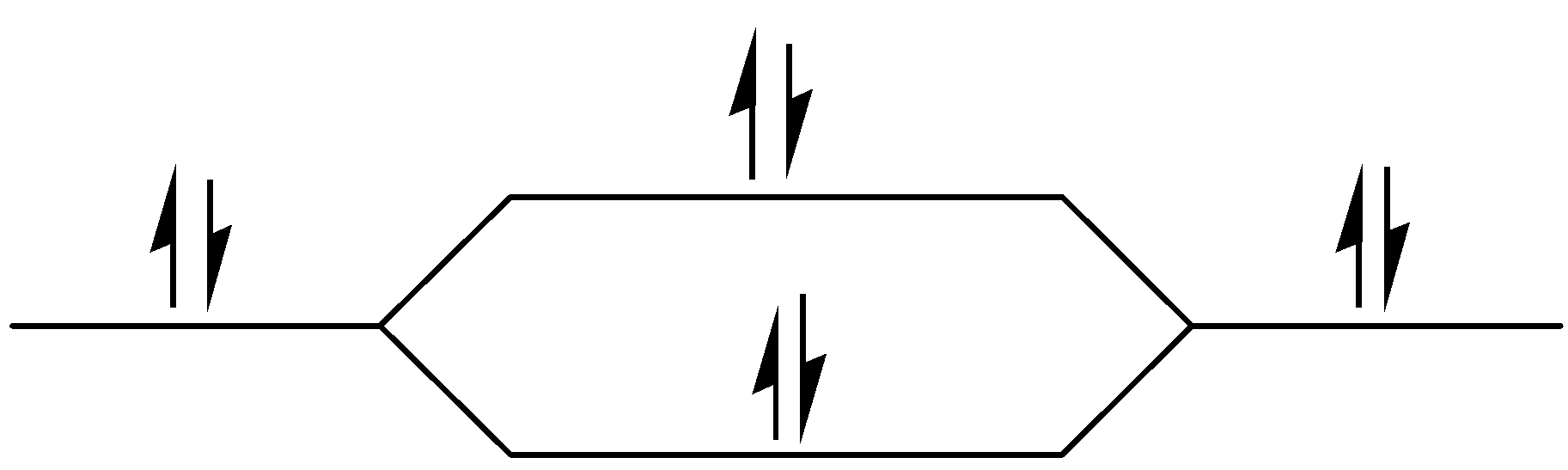
σ1s
Figure – The molecular orbital energy level diagram of N2 molecules
Let me explain the molecular orbital diagram of N2 using its diagram.
One atom of nitrogen has 7 electrons so a N2 molecule will have 14 electrons, so first 2 electrons go in 1s sigma bond, the next 2 in 1s sigma anti bond orbital, next 2 in 2s sigma bond orbital, next 2 in 2s sigma anti bond orbital, next 2 in 2pz sigma bond (assuming that z axis is the internuclear axis) orbital and next 4 in 2p pi x and 2 2p pi y orbitals
Number of bonding electrons:10 Number of anti-bonds: 4
bond order: 2(10−4)=3
This shows that N2 has a triple covalent bond. Since, all the electrons in nitrogen are paired, it is a diamagnetic molecule.
Note: Formation of molecular orbitals can be determined by LCAO (linear combination of atomic orbitals) method.
