Question
Question: Draw the molecular orbital diagram of \({N_2}\) and calculate the bond order....
Draw the molecular orbital diagram of N2 and calculate the bond order.
Solution
The molecular orbital theory is primarily used for explaining the type of bonding that exists in a molecule which cannot be explained by valence bond theory. The theory explains the geometry and bonding of the molecule which involve some form of resonance.
Complete answer: The basic rules of molecular orbital theory are as follows:
First principle: The number of molecular orbitals formed is always equal to the total number of atomic orbitals that have combined to form bonds.
Second principle: Bonding molecular orbitals always comprises lower energy as compared to antibonding molecular orbitals.
Third principle: Electrons of the molecule are always assigned to the orbitals in the increasing order of the orbital energy.
Fourth principle: atomic orbitals of similar energy combine to form most effective molecular orbitals.
The molecular orbital diagram of N2 is shown as follows:
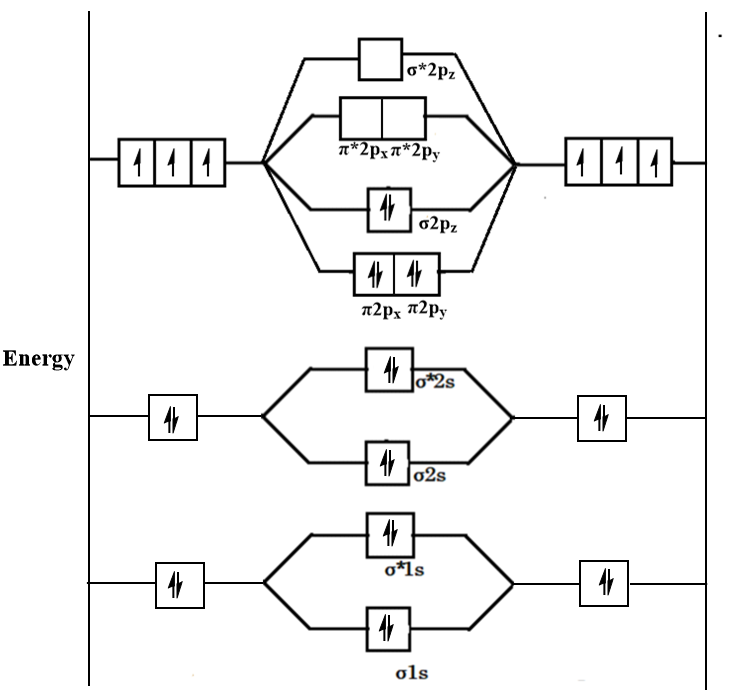
Now, the bond order of a molecule is determined by the formula which is as follows:
BO=2BMO−ABMO
Where, BMO represents the number of electrons present in the bonding molecular orbital and ABMO represents the number of electrons in antibonding molecular orbital.
For the given molecular orbital diagram, the value of BMO is 10 and the value of ABMO is 4. Therefore, the bond order will be as follows:
BO=210−4⇒3
Thus, we can conclude that the bond order of N2 is 3.
Note:
It is important to note that all the elements in the second period before oxygen consist of small energy difference between 2s and 2p orbitals so that he s-p mixing can occur lowering the energy of σ2s and σ∗2s and increase the energy of σ2p and σ∗2p. But on moving right in a period, the s-orbital becomes more stabilized than p-orbital and the difference in their energies increases due to which the s-p mixing of oxygen becomes smaller.
