Question
Question: Draw the lewis representation of \(S{O_2}\) molecule. Define dipole moment. The dipole moment of \(B...
Draw the lewis representation of SO2 molecule. Define dipole moment. The dipole moment of BF3 is zero. Why?
Solution
A Lewis structure of any molecule is based on the concept of the octet rule, in which atoms share electrons so that each atom has eight electrons in its outer shell.
Complete answer:
Lewis representation of SO2 molecule –
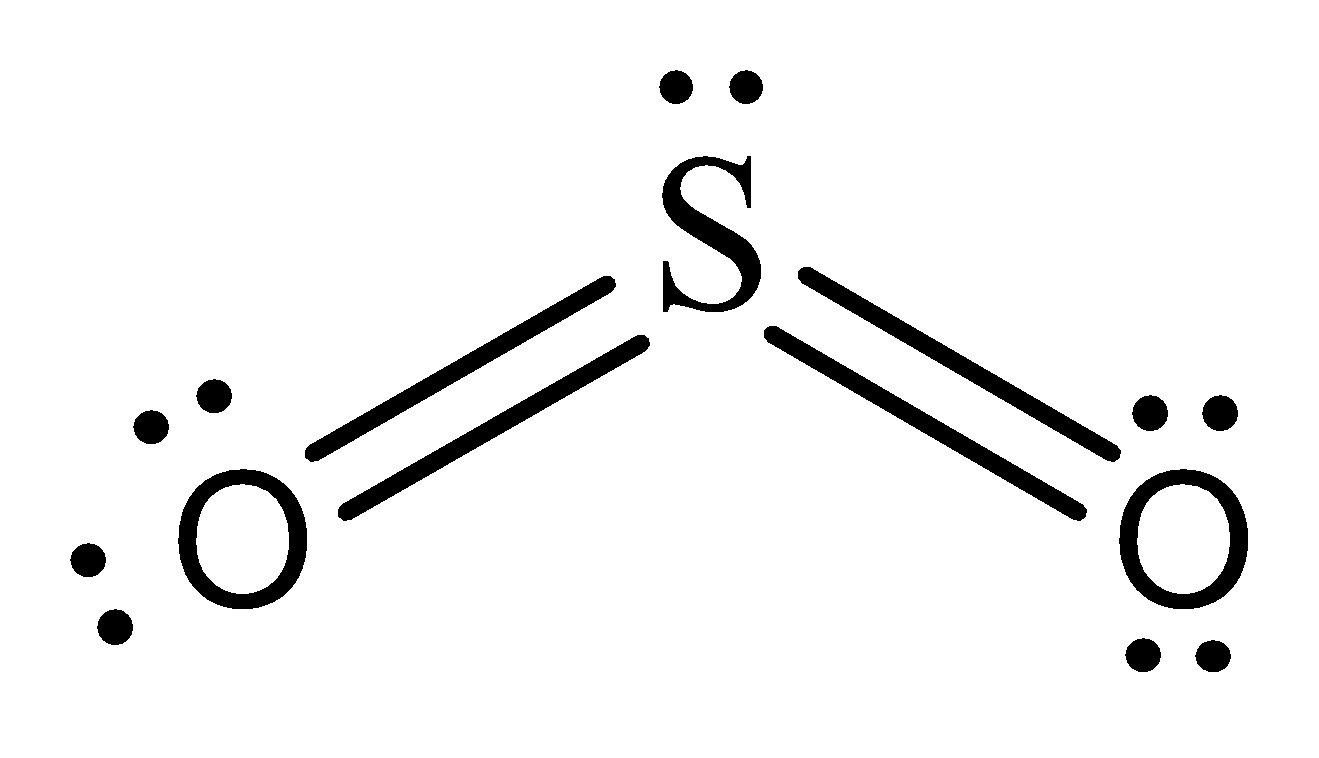
The formal charges of the SO2 with the single bond and a double bond is larger than SO2 with two double bonds.
A dipole moment arises in any system in which there is a separation of charge. They can, therefore, arise in ionic bonds as well as in covalent bonds. Dipole moments occur due to the difference in electronegativity between two chemically bonded atoms.
A bond dipole moment is a measure of the polarity of a chemical bond between two atoms in a molecule. It involves the concept of electric dipole moment, which is a measure of the separation of negative and positive charges in a system.
The BF3 molecule has a symmetrical trigonal planar geometry, like the SO3 molecule. In such a structure, the resultant moment of any two B−F dipoles is equal in magnitude but opposite in direction to the moment of the third one. So, the net dipole moment of the BF3 molecule is zero, and it is nonpolar.
In case of BF3 the dipole moment is zero because it has a regular geometry and no lone pair of electrons is present on B. All the angles are 120 degrees.
Note: The dipole moment is a vector quantity, that means it has magnitude as well as definite directions. And because of being a vector quantity, it can also be zero as the two oppositely acting bond dipoles can cancel each other. By convention, dipole moment is denoted by a small arrow with its tail on the negative centre and its head on the positive centre.
