Question
Question: Draw the Lewis electron dot structure of \(\text{S}{{\text{F}}_{\text{2}}}\). How many lone pairs of...
Draw the Lewis electron dot structure of SF2. How many lone pairs of electrons are on the central atom?
Solution
Lewis dot structure also called electron dot structure shows it valence electrons of elements and describes the chemical bonding. They also display the total number of lone presents in each of the atoms. Lewis dot structures can be drawn if the molecular formula of the compound is known.
It defines the nature of bond and position of atoms of the molecule which are connected in the molecule.
Complete step by step answer:
To draw a Lewis dot structure:
Total number of valence electrons present in the molecule in calculated
For SF2 → Sulphur (16)→2,8,5
Contains −6 valence electrons
Fluorine (9)→2,7
7− valence electrons
Total valence electrons −2×7+6
The least electronegative atom is made the central atom of a molecule.
For SF2→ Sulphur is the central atom
Atom are connected via single bonds
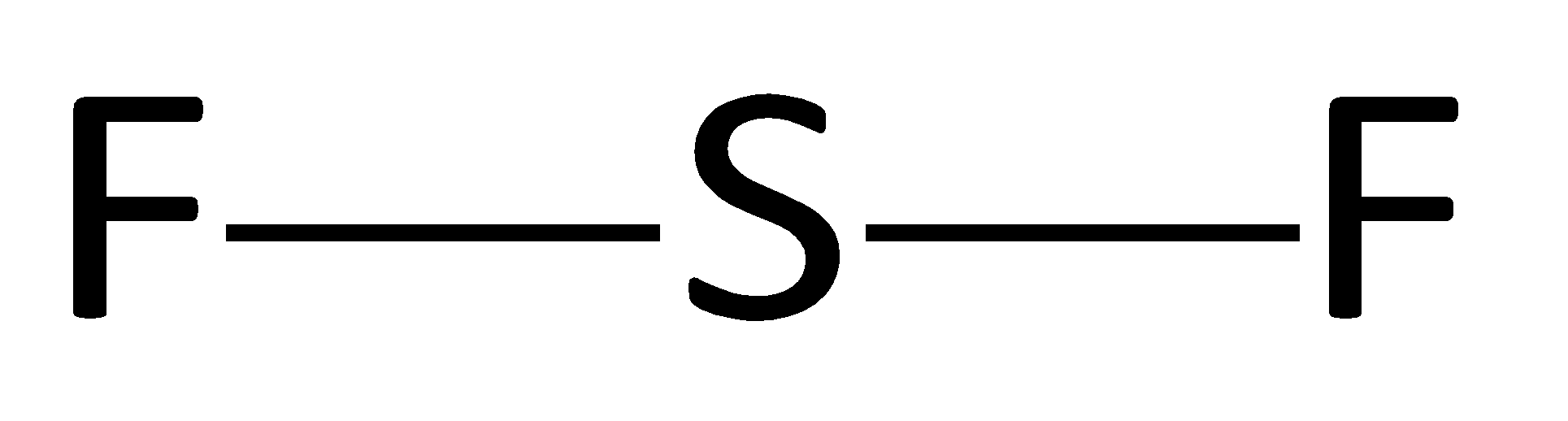
Lene pairs of e−are distributed. Firstly to the most electronegative element.
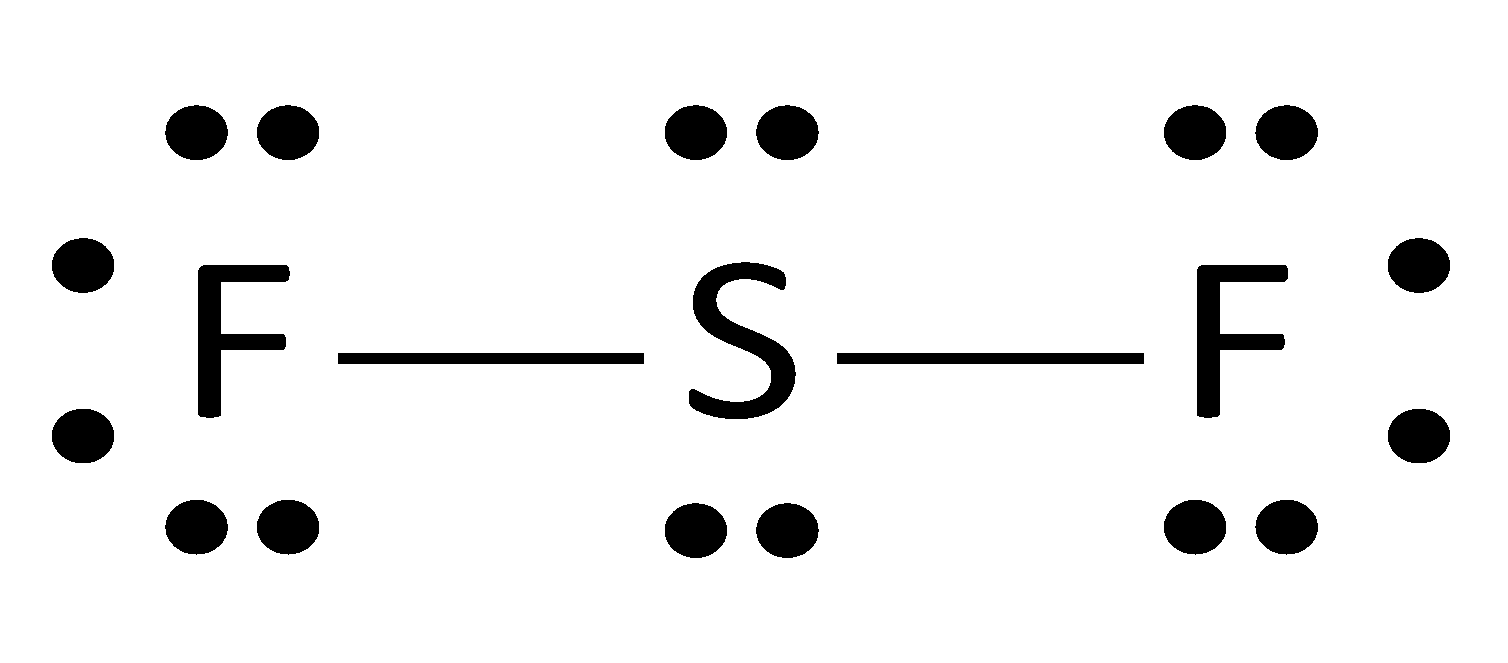
If the octet rule does not satisfy, double or triple bonds are drawn.
Here is the electron dot structure of SF2
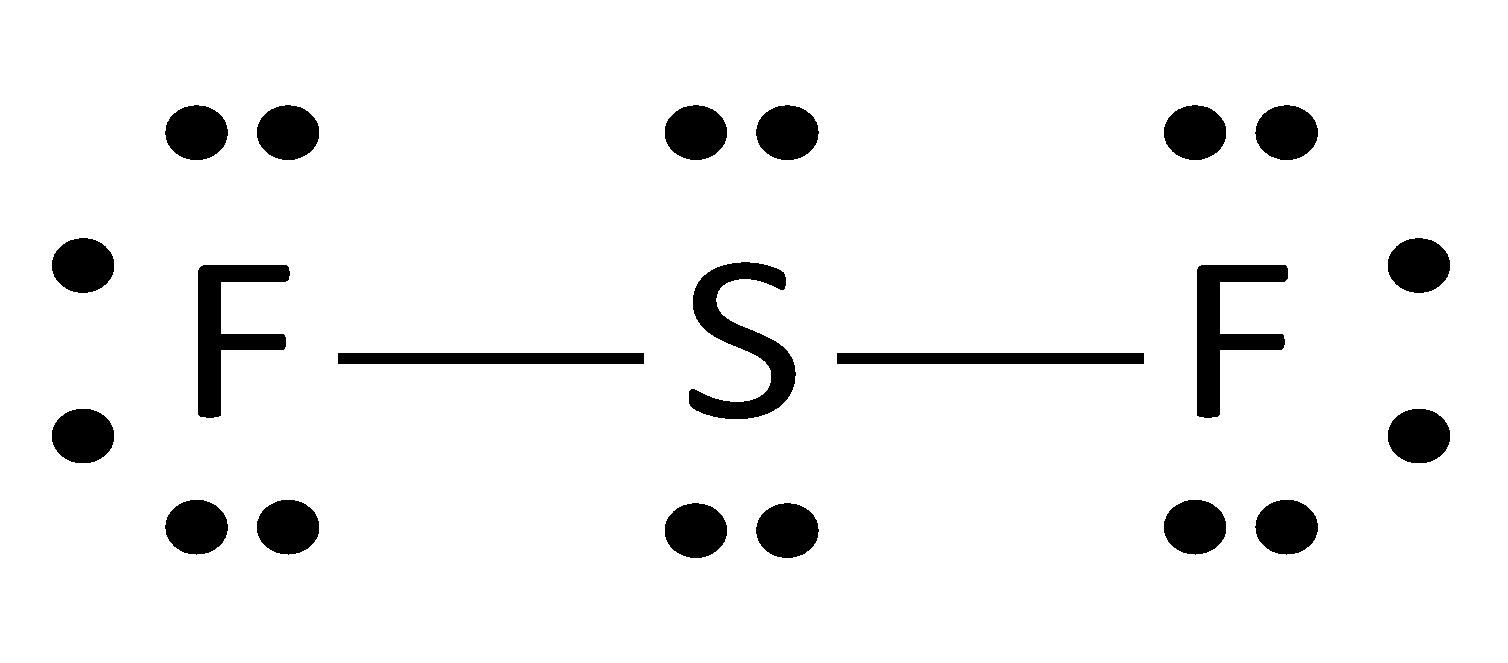
The central atom contains 2 lone pairs i.e., sulphur,
Note: Lewis defined a base as an electron pair donor and an acid as an electron pair acceptor
SF2 forms a covalent bond because sapphire and fluorine share their valence electrons to complete octet.
Nole that only the valence electrons are considered while drawing Lewis not structures and the electrons that do not belong to the outermost shell.
