Question
Question: Draw the Lewis dot structure of \(N{H}_{3}\). How many unshared pairs of electrons are in the outer ...
Draw the Lewis dot structure of NH3. How many unshared pairs of electrons are in the outer shell of the central nitrogen atom?
a) 0
b) 1
c) 2
d) 4
e) 6
Solution
A Lewis structure is defined as a very simplified representation of the valence shell electrons in a molecule. They show the bonding between the atoms of a molecule and the lone pairs of electrons that may exist in the molecule.
Complete step by step answer:
The Lewis dot structure are the diagrams that represent the valence electrons of atoms in a molecule. It is used to show how the electrons are arranged around individual atoms inside a molecule. In this structure, as the name suggests, electrons are shown as dots and a line for bonding electrons between the two atoms.
Let us now draw the Lewis dot structure of NH3. We know that nitrogen has five valence electrons in its outer shell and hydrogen has only one electron in its valence shell. Therefore, the individual atoms may look like this:
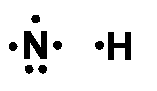
And when they are put together to form NH3, the Lewis structure looks like this:
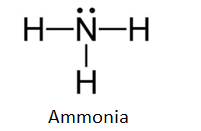
Now, if we look at the Lewis structure we can see that nitrogen has two unpaired electrons left or we can say that it has 1 lone pair of unshared electrons.
Hence, the correct option is option (b).
Note: Keep in mind that Lewis structure does not give you the proper structure or shape of the compound. It gives you the general idea of how the electrons are shared among atoms.
