Question
Question: Draw the geometry of \[OSF4\] using VSEPR theory....
Draw the geometry of OSF4 using VSEPR theory.
Solution
VSEPR stands for Valence shell electron pair repulsion.
This theory is used to predict molecular structures based on the number of electron pairs present around the central atom.
Complete step by step answer:
The basis of this theory lies in the fact that valence electrons of an atom repel each other hence they will try to obtain a conformation where the repulsive force between them will be the least. This is done to decrease the energy of the molecule thereby increasing its stability.
The number of valence electrons will be determined by drawing the Lewis structure of the molecule, showing all the bond pairs and the lone pairs of electrons. In VSEPR, a double or triple bond is also treated as a single bond.
In order to minimize the repulsions, electrons surrounding the central atom tend to move as far as possible from each other. Here is a set of examples of the total number of electron pairs around the central atom and the angles between them.
| No. of electron pairs around the central atom | Maximum possible angle between the electron pairs | Geometry of such a molecule |
|---|---|---|
| 2 | 180∘ | Linear |
| 3 | 120∘ | Trigonal planar |
| 4 | 109.5∘ | Tetrahedral |
| 5 | 90∘and120∘ | Trigonal bipyramidal |
| 6 | 90∘ | Octahedral |
| 7 | 90∘and72∘ | Pentagonal bipyramidal |
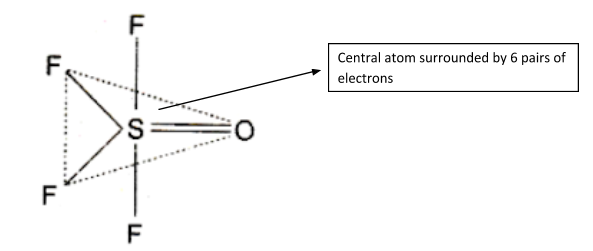
In OSF4, the number of electron pairs around S is 5. So, its geometry would be Trigonal Bipyramidal. This is how it would look:
Note:
It is important to consider the change in molecular shape if lone pairs are present.
