Question
Question: Draw the electron dot structures for: A.Ethanoic acid B.\({{\text{H}}_{\text{2}}}\text{S}\) C....
Draw the electron dot structures for:
A.Ethanoic acid
B.H2S
C.Propanone
D.F2
Solution
The electron dot structure, introduced by Lewis, is a representation of the valance electrons of an atom that uses dots around the symbol of the element. The shared pair of electrons which form bonds are drawn together in the overlapping circles drawn around the atom.
Complete step by step answer:
A.Ethanoic acid- commonly known by the name ‘acetic acid’, ethanoic acid has a carboxylic group in its molecule. The molecular formula of the compound is CH3COOH . The carbon atoms form four covalent bonds while the hydrogen atom one each and the oxygen atom forms two. The electron dot structure is:
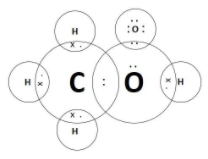
B.Hydrogen Sulphide has molecular formula H2S and the electron dot structure for the same is as follows:
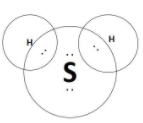
C.Propanone is commonly called as “acetone” and has the molecular formula CH3COCH3 . This compound has a double bond between the carbon and the oxygen atoms and the electron dot structure of the same is as follows:
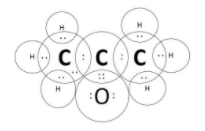
D.The final molecules is fluorine with molecular formula, F2
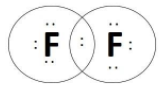
Note:
In the above figures, the overlapping areas depict the covalent bond where an electron pair is shared between the atoms. The electron pairs on the side of the atoms are called the lone pair or non-bonding electrons and the double bond in propanone is designated by two pairs of electrons.
