Question
Question: Draw the electron dot structure of \({{H}_{2}}S\) (hydrogen sulphide)?...
Draw the electron dot structure of H2S (hydrogen sulphide)?
Solution
The hybridisation of the central atom sulphur in hydrogen sulphide issp3. The two hydrogen atoms are attached to the central atom sulphur by sigma bonds, and sulphur has two lone pairs of electrons.
Complete answer:
The Lewis structure of H2S is very similar toH2O. Hydrogen is in Group 1 and therefore has only one valence electron. Hydrogen atoms only need 2 valence electrons to have a full outer shell. The Lewis structure forH2S has a total of 8 valence electrons.
Hydrogen has 1 valence electron, but we have two Hydrogens here. So, the total valence electrons of hydrogen are 2. Sulphur is in group 16 on the periodic table, so it has 6 valence electrons. Therefore, the total valence electrons of hydrogen sulphide are eight. Now, two valence electrons of sulphur bond with two hydrogens and we have four electrons remaining on sulphur which make up two lone pairs of electrons of sulphur. The Lewis dot structure of hydrogen sulphide is given below:
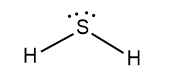
Hydrogen sulphide is a bent shaped molecule. Its molecular weight is 34g/mol. It is a colourless gas having a strong odour of rotten eggs. Its boiling point is−60.2∘C. It is a highly toxic and flammable gas. It is also heavier than air.
Note:
If the lone pair of electrons are not accounted for, a student might confuse the hybridization of H2S to be sp. The shape of the molecule in that case would come out to be linear.
